Dopamine-dependent tuning of striatal inhibitory synaptogenesis
- PMID: 20181591
- PMCID: PMC3930856
- DOI: 10.1523/JNEUROSCI.4411-09.2010
Dopamine-dependent tuning of striatal inhibitory synaptogenesis
Abstract
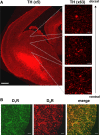
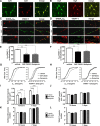
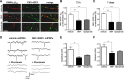
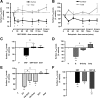
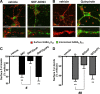
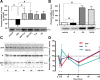
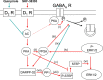
Dopaminergic projections to the striatum, crucial for the correct functioning of this brain region in adulthood, are known to be established early in development, but their role is currently uncharacterized. We demonstrate here that dopamine, by activating D(1)- and/or D(2)-dopamine receptors, decreases the number of functional GABAergic synapses formed between the embryonic precursors of the medium spiny neurons, the principal output neurons of the striatum, with associated changes in spontaneous synaptic activity. Activation of these receptors reduces the size of postsynaptic GABA(A) receptor clusters and their overall cell-surface expression, without affecting the total number of clusters or the size or number of GABAergic nerve terminals. These changes result from an increased internalization of GABA(A) receptors, and are mediated by distinct signaling pathways converging at the level of GABA(A) receptors to cause a transient PP2A/PP1-dependent dephosphorylation. Thus, tonic D(1)- and D(2)-receptor activity limits the extent of collateral inhibitory synaptogenesis between medium spiny neurons, revealing a novel role of dopamine in controlling the development of intrinsic striatal microcircuits.
Figures

Similar articles
-
Dopamine presynaptically and heterogeneously modulates nucleus accumbens medium-spiny neuron GABA synapses in vitro.BMC Neurosci. 2006 Jun 30;7:53. doi: 10.1186/1471-2202-7-53. BMC Neurosci. 2006. PMID: 16813648 Free PMC article.
-
Alterations in nigral NMDA and GABAA receptor control of the striatal dopamine level after repetitive exposures to nitrogen narcosis.Exp Neurol. 2008 Jul;212(1):63-70. doi: 10.1016/j.expneurol.2008.03.001. Epub 2008 Mar 16. Exp Neurol. 2008. PMID: 18452916
-
The role of NMDA and GABAA receptors in the inhibiting effect of 3 MPa nitrogen on striatal dopamine level.Brain Res. 2007 Oct 24;1176:37-44. doi: 10.1016/j.brainres.2007.07.085. Epub 2007 Aug 22. Brain Res. 2007. PMID: 17900538
-
GABAergic signaling in the developing cerebellum.Anat Sci Int. 2004 Sep;79(3):124-36. doi: 10.1111/j.1447-073x.2004.00081.x. Anat Sci Int. 2004. PMID: 15453613 Review.
-
Localization and pharmacology of some dopamine receptor complexes in the striatum and the pituitary gland: synaptic and son-synaptic communication.Acta Morphol Neerl Scand. 1988-1989;26(2-3):115-30. Acta Morphol Neerl Scand. 1988. PMID: 2855385 Review.
Cited by
-
Regulation of GABAARs by phosphorylation.Adv Pharmacol. 2015;72:97-146. doi: 10.1016/bs.apha.2014.11.008. Epub 2014 Dec 19. Adv Pharmacol. 2015. PMID: 25600368 Free PMC article. Review.
-
Alteration of GABAergic neurotransmission in Huntington's disease.CNS Neurosci Ther. 2018 Apr;24(4):292-300. doi: 10.1111/cns.12826. Epub 2018 Feb 21. CNS Neurosci Ther. 2018. PMID: 29464851 Free PMC article. Review.
-
Differential Alteration in Expression of Striatal GABAAR Subunits in Mouse Models of Huntington's Disease.Front Mol Neurosci. 2017 Jun 20;10:198. doi: 10.3389/fnmol.2017.00198. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28676743 Free PMC article.
-
Metformin Prevented Dopaminergic Neurotoxicity Induced by 3,4-Methylenedioxymethamphetamine Administration.Neurotox Res. 2016 Jul;30(1):101-9. doi: 10.1007/s12640-016-9633-5. Epub 2016 Jun 1. Neurotox Res. 2016. PMID: 27251371
-
Characterization of the Functional Cross-Talk between Surface GABAA and Dopamine D5 Receptors.Int J Mol Sci. 2021 May 4;22(9):4867. doi: 10.3390/ijms22094867. Int J Mol Sci. 2021. PMID: 34064454 Free PMC article.
References
-
- Aizman O, Brismar H, Uhlén P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–230. - PubMed
-
- Awad JA, Johnson RA, Jakobs KH, Schultz G. Interactions of forskolin and adenylate cyclase. Effects on substrate kinetics and protection against inactivation by heat and N-ethylmaleimide. J Biol Chem. 1983;258:2960–2965. - PubMed
-
- Bedford FK, Kittler JT, Muller E, Thomas P, Uren JM, Merlo D, Wisden W, Triller A, Smart TG, Moss SJ. GABA(A) receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neurosci. 2001;4:908–916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
