Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury
- PMID: 20181596
- PMCID: PMC2836860
- DOI: 10.1523/JNEUROSCI.3174-09.2010
Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury
Abstract
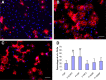
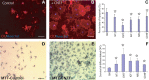
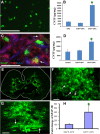
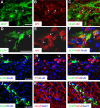
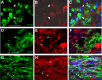
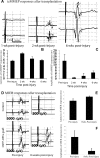
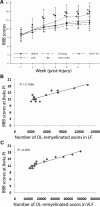
Demyelination contributes to the dysfunction after traumatic spinal cord injury (SCI). We explored whether the combination of neurotrophic factors and transplantation of adult rat spinal cord oligodendrocyte precursor cells (OPCs) could enhance remyelination and functional recovery after SCI. Ciliary neurotrophic factor (CNTF) was the most effective neurotrophic factor to promote oligodendrocyte (OL) differentiation and survival of OPCs in vitro. OPCs were infected with retroviruses expressing enhanced green fluorescent protein (EGFP) or CNTF and transplanted into the contused adult thoracic spinal cord 9 d after injury. Seven weeks after transplantation, the grafted OPCs survived and integrated into the injured spinal cord. The survival of grafted CNTF-OPCs increased fourfold compared with EGFP-OPCs. The grafted OPCs differentiated into adenomatus polyposis coli (APC(+)) OLs, and CNTF significantly increased the percentage of APC(+) OLs from grafted OPCs. Immunofluorescent and immunoelectron microscopic analyses showed that the grafted OPCs formed central myelin sheaths around the axons in the injured spinal cord. The number of OL-remyelinated axons in ventrolateral funiculus (VLF) or lateral funiculus (LF) at the injured epicenter was significantly increased in animals that received CNTF-OPC grafts compared with all other groups. Importantly, 75% of rats receiving CNTF-OPC grafts recovered transcranial magnetic motor-evoked potential and magnetic interenlargement reflex responses, indicating that conduction through the demyelinated axons in VLF or LF, respectively, was partially restored. More importantly, recovery of hindlimb locomotor function was significantly enhanced in animals receiving grafts of CNTF-OPCs. Thus, combined treatment with OPC grafts expressing CNTF can enhance remyelination and facilitate functional recovery after traumatic SCI.
Figures


Similar articles
-
Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells.J Neurosci. 2005 Jul 27;25(30):6947-57. doi: 10.1523/JNEUROSCI.1065-05.2005. J Neurosci. 2005. PMID: 16049170 Free PMC article.
-
Transplantation of oligodendrocyte precursor cells improves locomotion deficits in rats with spinal cord irradiation injury.PLoS One. 2013;8(2):e57534. doi: 10.1371/journal.pone.0057534. Epub 2013 Feb 27. PLoS One. 2013. PMID: 23460872 Free PMC article.
-
Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury.J Neurosci. 2006 Mar 29;26(13):3377-89. doi: 10.1523/JNEUROSCI.4184-05.2006. J Neurosci. 2006. PMID: 16571744 Free PMC article.
-
Engineering biomaterial microenvironments to promote myelination in the central nervous system.Brain Res Bull. 2019 Oct;152:159-174. doi: 10.1016/j.brainresbull.2019.07.013. Epub 2019 Jul 12. Brain Res Bull. 2019. PMID: 31306690 Review.
-
The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury.Glia. 2020 Feb;68(2):227-245. doi: 10.1002/glia.23706. Epub 2019 Aug 21. Glia. 2020. PMID: 31433109 Review.
Cited by
-
Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury.J Neurotrauma. 2011 Apr;28(4):579-94. doi: 10.1089/neu.2010.1626. Epub 2011 Mar 24. J Neurotrauma. 2011. PMID: 21222572 Free PMC article.
-
What is the potential of oligodendrocyte progenitor cells to successfully treat human spinal cord injury?BMC Neurol. 2011 Sep 23;11:113. doi: 10.1186/1471-2377-11-113. BMC Neurol. 2011. PMID: 21943254 Free PMC article. Review.
-
The cancer drug tamoxifen: a potential therapeutic treatment for spinal cord injury.J Neurotrauma. 2014 Feb 1;31(3):268-83. doi: 10.1089/neu.2013.3108. Epub 2013 Dec 11. J Neurotrauma. 2014. PMID: 24004276 Free PMC article.
-
Topographical effects on fiber-mediated microRNA delivery to control oligodendroglial precursor cells development.Biomaterials. 2015 Nov;70:105-14. doi: 10.1016/j.biomaterials.2015.08.029. Epub 2015 Aug 18. Biomaterials. 2015. PMID: 26310106 Free PMC article.
-
Stem cell transplantation for spinal cord injury: a meta-analysis of treatment effectiveness and safety.Neural Regen Res. 2017 May;12(5):815-825. doi: 10.4103/1673-5374.206653. Neural Regen Res. 2017. PMID: 28616040 Free PMC article.
References
-
- Alexeeva N, Broton JG, Suys S, Calancie B. Central cord syndrome of cervical spinal cord injury: widespread changes in muscle recruitment studied by voluntary contractions and transcranial magnetic stimulation. Exp Neurol. 1997;148:399–406. - PubMed
-
- Alexeeva N, Broton JG, Calancie B. Latency of changes in spinal motoneuron excitability evoked by transcranial magnetic brain stimulation in spinal cord injured individuals. Electroencephalogr Clin Neurophysiol. 1998;109:297–303. - PubMed
-
- Bambakidis NC, Miller RH. Transplantation of oligodendrocyte precursors and sonic hedgehog results in improved function and white matter sparing in the spinal cords of adult rats after contusion. Spine J. 2004;4:16–26. - PubMed
-
- Barbin G, Manthorpe M, Varon S. Purification of the chick eye ciliary neuronotrophic factor. J Neurochem. 1984;43:1468–1478. - PubMed
-
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
