Novel embryonic neuronal migration and proliferation defects in Dcx mutant mice are exacerbated by Lis1 reduction
- PMID: 20181597
- PMCID: PMC2861429
- DOI: 10.1523/JNEUROSCI.4851-09.2010
Novel embryonic neuronal migration and proliferation defects in Dcx mutant mice are exacerbated by Lis1 reduction
Abstract
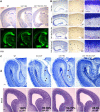
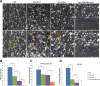
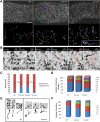
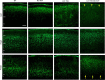
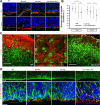
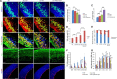
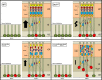
Heterozygous LIS1 mutations and males with loss of the X-linked DCX result in lissencephaly, a neuronal migration defect. LIS1 regulates nuclear translocation and mitotic division of neural progenitor cells, while the role of DCX in cortical development remains poorly understood. Here, we uncovered novel neuronal migration and proliferation defects in the Dcx mutant embryonic brains. Although cortical organization was fairly well preserved, Dcx(ko/Y) neurons displayed defective migration velocities similar to Lis1(+/ko) neurons when characterized by time-lapse video-microscopy of embryonic cortical slices. Dcx(ko/Y) migrating neurons displayed novel multidirectional movements with abnormal morphology and increased branching. Surprisingly, Dcx(ko/Y) radial glial cells displayed spindle orientation abnormalities similar to Lis1(+/ko) cells that in turn lead to moderate proliferation defects both in vivo and in vitro. We found functional genetic interaction of the two genes, with the combined effects of Lis1 haploinsufficiency and Dcx knock-out leading to more severe neuronal migration and proliferation phenotypes in the Lis1(+/ko);Dcx(ko/Y) male double mutant compared with the single mutants, resulting in cortical disorganization and depletion of the progenitor pool. Thus, we provide definitive evidence for a critical role for Dcx in neuronal migration and neurogenesis, as well as for the in vivo genetic interaction of the two genes most commonly involved in human neuronal migration defects.
Figures
Similar articles
-
Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration.J Cell Biol. 2004 Jun 7;165(5):709-21. doi: 10.1083/jcb.200309025. Epub 2004 Jun 1. J Cell Biol. 2004. PMID: 15173193 Free PMC article.
-
Global developmental gene expression and pathway analysis of normal brain development and mouse models of human neuronal migration defects.PLoS Genet. 2011 Mar;7(3):e1001331. doi: 10.1371/journal.pgen.1001331. Epub 2011 Mar 10. PLoS Genet. 2011. PMID: 21423666 Free PMC article.
-
Distinct dose-dependent cortical neuronal migration and neurite extension defects in Lis1 and Ndel1 mutant mice.J Neurosci. 2009 Dec 9;29(49):15520-30. doi: 10.1523/JNEUROSCI.4630-09.2009. J Neurosci. 2009. PMID: 20007476 Free PMC article.
-
Lissencephaly and LIS1: insights into the molecular mechanisms of neuronal migration and development.Clin Genet. 2007 Oct;72(4):296-304. doi: 10.1111/j.1399-0004.2007.00888.x. Clin Genet. 2007. PMID: 17850624 Review.
-
LIS1 functions in normal development and disease.Curr Opin Neurobiol. 2013 Dec;23(6):951-6. doi: 10.1016/j.conb.2013.08.001. Epub 2013 Aug 23. Curr Opin Neurobiol. 2013. PMID: 23973156 Review.
Cited by
-
Fine-tuning of neurogenesis is essential for the evolutionary expansion of the cerebral cortex.Cereb Cortex. 2015 Feb;25(2):346-64. doi: 10.1093/cercor/bht232. Epub 2013 Aug 22. Cereb Cortex. 2015. PMID: 23968831 Free PMC article.
-
Cancer stem cell contribution to glioblastoma invasiveness.Stem Cell Res Ther. 2013 Feb 28;4(1):18. doi: 10.1186/scrt166. Stem Cell Res Ther. 2013. PMID: 23510696 Free PMC article. Review.
-
Causes and consequences of gray matter heterotopia.CNS Neurosci Ther. 2015 Feb;21(2):112-22. doi: 10.1111/cns.12322. Epub 2014 Sep 2. CNS Neurosci Ther. 2015. PMID: 25180909 Free PMC article. Review.
-
Loss of Usp9x disrupts cortical architecture, hippocampal development and TGFβ-mediated axonogenesis.PLoS One. 2013 Jul 5;8(7):e68287. doi: 10.1371/journal.pone.0068287. Print 2013. PLoS One. 2013. PMID: 23861879 Free PMC article.
-
Increasing doublecortin expression promotes migration of human embryonic stem cell-derived neurons.Stem Cells. 2012 Sep;30(9):1852-62. doi: 10.1002/stem.1162. Stem Cells. 2012. PMID: 22753232 Free PMC article.
References
-
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–1283. - PubMed
-
- Bantubungi K, Blum D, Cuvelier L, Wislet-Gendebien S, Rogister B, Brouillet E, Schiffmann SN. Stem cell factor and mesenchymal and neural stem cell transplantation in a rat model of Huntington's disease. Mol Cell Neurosci. 2008;37:454–470. - PubMed
-
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. - PubMed
-
- Caspi M, Atlas R, Kantor A, Sapir T, Reiner O. Interaction between LIS1 and doublecortin, two lissencephaly gene products. Hum Mol Genet. 2000;9:2205–2213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
