Macaque parieto-insular vestibular cortex: responses to self-motion and optic flow
- PMID: 20181599
- PMCID: PMC3108058
- DOI: 10.1523/JNEUROSCI.4029-09.2010
Macaque parieto-insular vestibular cortex: responses to self-motion and optic flow
Abstract
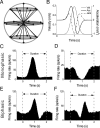
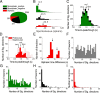
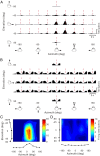
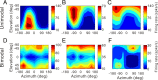
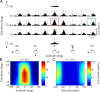
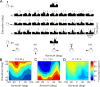
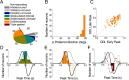
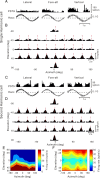
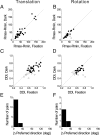
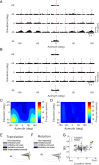
The parieto-insular vestibular cortex (PIVC) is thought to contain an important representation of vestibular information. Here we describe responses of macaque PIVC neurons to three-dimensional (3D) vestibular and optic flow stimulation. We found robust vestibular responses to both translational and rotational stimuli in the retroinsular (Ri) and adjacent secondary somatosensory (S2) cortices. PIVC neurons did not respond to optic flow stimulation, and vestibular responses were similar in darkness and during visual fixation. Cells in the upper bank and tip of the lateral sulcus (Ri and S2) responded to sinusoidal vestibular stimuli with modulation at the first harmonic frequency and were directionally tuned. Cells in the lower bank of the lateral sulcus (mostly Ri) often modulated at the second harmonic frequency and showed either bimodal spatial tuning or no tuning at all. All directions of 3D motion were represented in PIVC, with direction preferences distributed approximately uniformly for translation, but showing a preference for roll rotation. Spatiotemporal profiles of responses to translation revealed that half of PIVC cells followed the linear velocity profile of the stimulus, one-quarter carried signals related to linear acceleration (in the form of two peaks of direction selectivity separated in time), and a few neurons followed the derivative of linear acceleration (jerk). In contrast, mainly velocity-coding cells were found in response to rotation. Thus, PIVC comprises a large functional region in macaque areas Ri and S2, with robust responses to 3D rotation and translation, but is unlikely to play a significant role in visual/vestibular integration for self-motion perception.
Figures
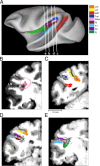
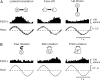
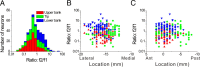
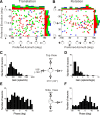

References
-
- Akbarian S, Berndl K, Grusser OJ, Guldin W, Pause M, Schreiter U. Responses of single neurons in the parietoinsular vestibular cortex of primates. Ann N Y Acad Sci. 1988;545:187–202. - PubMed
-
- Akbarian S, Grüsser OJ, Guldin WO. Thalamic connections of the vestibular cortical fields in the squirrel monkey (Saimiri sciureus) J Comp Neurol. 1992;326:423–441. - PubMed
-
- Akbarian S, Grüsser OJ, Guldin WO. Corticofugal connections between the cerebral cortex and brainstem vestibular nuclei in the macaque monkey. J Comp Neurol. 1994;339:421–437. - PubMed
-
- Angelaki DE. Dynamic polarization vector of spatially tuned neurons. IEEE Trans Biomed Eng. 1991;38:1053–1060. - PubMed
-
- Angelaki DE. Spatio-temporal convergence (STC) in otolith neurons. Biol Cybern. 1992;67:83–96. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
