Bral1: its role in diffusion barrier formation and conduction velocity in the CNS
- PMID: 20181608
- PMCID: PMC6633924
- DOI: 10.1523/JNEUROSCI.5598-09.2010
Bral1: its role in diffusion barrier formation and conduction velocity in the CNS
Abstract
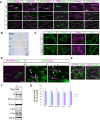
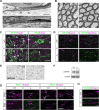
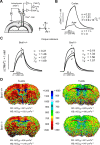
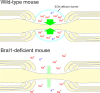
At the nodes of Ranvier, excitable axon membranes are exposed directly to the extracellular fluid. Cations are accumulated and depleted in the local extracellular nodal region during action potential propagation, but the impact of the extranodal micromilieu on signal propagation still remains unclear. Brain-specific hyaluronan-binding link protein, Bral1, colocalizes and forms complexes with negatively charged extracellular matrix (ECM) proteins, such as versican V2 and brevican, at the nodes of Ranvier in the myelinated white matter. The link protein family, including Bral1, appears to be the linchpin of these hyaluronan-bound ECM complexes. Here we report that the hyaluronan-associated ECM no longer shows a nodal pattern and that CNS nerve conduction is markedly decreased in Bral1-deficient mice even though there were no differences between wild-type and mutant mice in the clustering or transition of ion channels at the nodes or in the tissue morphology around the nodes of Ranvier. However, changes in the extracellular space diffusion parameters, measured by the real-time iontophoretic method and diffusion-weighted magnetic resonance imaging (MRI), suggest a reduction in the diffusion hindrances in the white matter of mutant mice. These findings provide a better understanding of the mechanisms underlying the accumulation of cations due to diffusion barriers around the nodes during saltatory conduction, which further implies the importance of the Bral1-based extramilieu for neuronal conductivity.
Figures



References
-
- Asher RA, Scheibe RJ, Keiser HD, Bignami A. On the existence of a cartilage-like proteoglycan and link proteins in the central nervous system. Glia. 1995;13:294–308. - PubMed
-
- Aspberg A, Miura R, Bourdoulous S, Shimonaka M, Heinegârd D, Schachner M, Ruoslahti E, Yamaguchi Y. The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein-protein interactions independent of carbohydrate moiety. Proc Natl Acad Sci U S A. 1997;94:10116–10121. - PMC - PubMed
-
- Bekku Y, Su WD, Hirakawa S, Fässler R, Ohtsuka A, Kang JS, Sanders J, Murakami T, Ninomiya Y, Oohashi T. Molecular cloning of Bral2, a novel brain-specific link protein, and immunohistochemical colocalization with brevican in perineuronal nets. Mol Cell Neurosci. 2003;24:148–159. - PubMed
-
- Bekku Y, Rauch U, Ninomiya Y, Oohashi T. Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. J Neurochem. 2009;108:1266–1276. - PubMed
-
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
