Heart-type fatty acid binding protein regulates dopamine D2 receptor function in mouse brain
- PMID: 20181611
- PMCID: PMC6633935
- DOI: 10.1523/JNEUROSCI.4140-09.2010
Heart-type fatty acid binding protein regulates dopamine D2 receptor function in mouse brain
Abstract
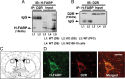
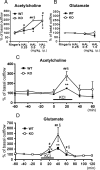
Fatty acid binding proteins (FABPs) are essential for energy production and long-chain polyunsaturated fatty acid-related signaling in the brain and other tissues. Of various FABPs, heart-type fatty acid binding protein (H-FABP, FABP3) is highly expressed in neurons of mature brain and plays a role in arachidonic acid incorporation into brain and heart cells. However, the precise function of H-FABP in brain remains unclear. We previously demonstrated that H-FABP is associated with the dopamine D(2) receptor long isoform (D2LR) in vitro. Here, we confirm that H-FABP binds to dopamine D(2) receptor (D2R) in brain extracts and colocalizes immunohistochemically with D2R in the dorsal striatum. We show that H-FABP is highly expressed in acetylcholinergic interneurons and terminals of glutamatergic neurons in the dorsal striatum of mouse brain but absent in dopamine neuron terminals and spines in the same region. H-FABP knock-out (KO) mice showed lower responsiveness to methamphetamine-induced sensitization and enhanced haloperidol-induced catalepsy compared with wild-type mice, indicative of D2R dysfunction. Consistent with the latter, aberrant increased acetylcholine (ACh) release and depolarization-induced glutamate (Glu) release were observed in the dorsal striatum of H-FABP KO mice. Furthermore, phosphorylation of CaMKII (Ca(2+)/calmodulin-dependent protein kinase II) and ERK (extracellular signal-regulated kinase) was significantly increased in the dorsal striatum. We confirmed elevated ERK phosphorylation following quinpirole-mediated D2R stimulation in H-FABP-overexpressing SHSY-5Y human neuroblastoma cells. Together, H-FABP is highly expressed in ACh interneurons and glutamatergic terminals, thereby regulating dopamine D2R function in the striatum.
Figures





Similar articles
-
Endocytosis following dopamine D2 receptor activation is critical for neuronal activity and dendritic spine formation via Rabex-5/PDGFRβ signaling in striatopallidal medium spiny neurons.Mol Psychiatry. 2017 Aug;22(8):1205-1222. doi: 10.1038/mp.2016.200. Epub 2016 Dec 6. Mol Psychiatry. 2017. PMID: 27922607
-
[Regulation of dopaminergic neuronal activity by heart-type fatty acid binding protein in the brain].Yakugaku Zasshi. 2011 Apr;131(4):497-501. doi: 10.1248/yakushi.131.497. Yakugaku Zasshi. 2011. PMID: 21467787 Review. Japanese.
-
Impaired Acquisition of Nicotine-Induced Conditioned Place Preference in Fatty Acid-Binding Protein 3 Null Mice.Mol Neurobiol. 2021 May;58(5):2030-2045. doi: 10.1007/s12035-020-02228-2. Epub 2021 Jan 7. Mol Neurobiol. 2021. PMID: 33411237
-
Differential subcellular localization of two dopamine D2 receptor isoforms in transfected NG108-15 cells.J Neurochem. 2003 May;85(4):1064-74. doi: 10.1046/j.1471-4159.2003.01763.x. J Neurochem. 2003. PMID: 12716438
-
[Role of heart-type fatty acid binding protein in the brain function].Yakugaku Zasshi. 2009 Feb;129(2):191-5. doi: 10.1248/yakushi.129.191. Yakugaku Zasshi. 2009. PMID: 19182446 Review. Japanese.
Cited by
-
Diacylglycerol kinase β knockout mice exhibit attention-deficit behavior and an abnormal response on methylphenidate-induced hyperactivity.PLoS One. 2012;7(5):e37058. doi: 10.1371/journal.pone.0037058. Epub 2012 May 10. PLoS One. 2012. PMID: 22590645 Free PMC article.
-
Expression of a truncated form of the endoplasmic reticulum chaperone protein, σ1 receptor, promotes mitochondrial energy depletion and apoptosis.J Biol Chem. 2012 Jul 6;287(28):23318-31. doi: 10.1074/jbc.M112.349142. Epub 2012 May 22. J Biol Chem. 2012. PMID: 22619170 Free PMC article.
-
Fatty acid binding proteins are novel modulators of synaptic epoxyeicosatrienoic acid signaling in the brain.Sci Rep. 2023 Sep 14;13(1):15234. doi: 10.1038/s41598-023-42504-4. Sci Rep. 2023. PMID: 37709856 Free PMC article.
-
Endocytosis following dopamine D2 receptor activation is critical for neuronal activity and dendritic spine formation via Rabex-5/PDGFRβ signaling in striatopallidal medium spiny neurons.Mol Psychiatry. 2017 Aug;22(8):1205-1222. doi: 10.1038/mp.2016.200. Epub 2016 Dec 6. Mol Psychiatry. 2017. PMID: 27922607
-
Functional characterization of FABP3, 5 and 7 gene variants identified in schizophrenia and autism spectrum disorder and mouse behavioral studies.Hum Mol Genet. 2014 Dec 15;23(24):6495-511. doi: 10.1093/hmg/ddu369. Epub 2014 Jul 15. Hum Mol Genet. 2014. PMID: 25027319 Free PMC article.
References
-
- Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP. Supplementation with a combination of omega-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr Res. 2003a;62:195–204. - PubMed
-
- Arvindakshan M, Sitasawad S, Debsikdar V, Ghate M, Evans D, Horrobin DF, Bennett C, Ranjekar PK, Mahadik SP. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biol Psychiatry. 2003b;53:56–64. - PubMed
-
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
