In vivo targeting of B-cell lymphoma with glycan ligands of CD22
- PMID: 20181615
- PMCID: PMC2890185
- DOI: 10.1182/blood-2009-12-257386
In vivo targeting of B-cell lymphoma with glycan ligands of CD22
Erratum in
- Blood. 2011 May 19;117(20):5551
Abstract
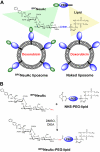
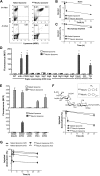
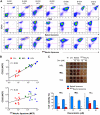
Antibody-mediated cell depletion therapy has proven to provide significant clinical benefit in treatment of lymphomas and leukemias, driving the development of improved therapies with novel mechanisms of cell killing. A current clinical target for B-cell lymphoma is CD22, a B-cell-specific member of the sialic acid binding Ig-like lectin (siglec) family that recognizes alpha2-6-linked sialylated glycans as ligands. Here, we describe a novel approach for targeting B lymphoma cells with doxorubicin-loaded liposomal nanoparticles displaying high-affinity glycan ligands of CD22. The targeted liposomes are actively bound and endocytosed by CD22 on B cells, and significantly extend life in a xenograft model of human B-cell lymphoma. Moreover, they bind and kill malignant B cells from peripheral blood samples obtained from patients with hairy cell leukemia, marginal zone lymphoma, and chronic lymphocytic leukemia. The results demonstrate the potential for using a carbohydrate recognition-based approach for efficiently targeting B cells in vivo that can offer improved treatment options for patients with B-cell malignancies.
Figures

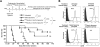
Comment in
-
Hitting the sweet spot for lymphoma.Blood. 2010 Jun 10;115(23):4626-7. doi: 10.1182/blood-2010-03-272955. Blood. 2010. PMID: 20538811 No abstract available.
-
Liposomes modified by carbohydrate ligands can target B cells for the treatment of B-cell lymphomas.Expert Rev Vaccines. 2010 Nov;9(11):1251-6. doi: 10.1586/erv.10.121. Expert Rev Vaccines. 2010. PMID: 21087105 Free PMC article.
Similar articles
-
Targeting B lymphoma with nanoparticles bearing glycan ligands of CD22.Leuk Lymphoma. 2012 Feb;53(2):208-10. doi: 10.3109/10428194.2011.604755. Epub 2011 Aug 24. Leuk Lymphoma. 2012. PMID: 21756025 Free PMC article. Review.
-
Glycoengineering of NK Cells with Glycan Ligands of CD22 and Selectins for B-Cell Lymphoma Therapy.Angew Chem Int Ed Engl. 2021 Feb 15;60(7):3603-3610. doi: 10.1002/anie.202005934. Epub 2020 Dec 14. Angew Chem Int Ed Engl. 2021. PMID: 33314603 Free PMC article.
-
CD22 Ligands on a Natural N-Glycan Scaffold Efficiently Deliver Toxins to B-Lymphoma Cells.J Am Chem Soc. 2017 Sep 13;139(36):12450-12458. doi: 10.1021/jacs.7b03208. Epub 2017 Aug 31. J Am Chem Soc. 2017. PMID: 28829594 Free PMC article.
-
CD22 is a recycling receptor that can shuttle cargo between the cell surface and endosomal compartments of B cells.J Immunol. 2011 Feb 1;186(3):1554-63. doi: 10.4049/jimmunol.1003005. Epub 2010 Dec 22. J Immunol. 2011. PMID: 21178016 Free PMC article.
-
Targeting CD22 in B-cell malignancies: current status and clinical outlook.BioDrugs. 2013 Aug;27(4):293-304. doi: 10.1007/s40259-013-0016-7. BioDrugs. 2013. PMID: 23696252 Review.
Cited by
-
Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169.PLoS One. 2012;7(6):e39039. doi: 10.1371/journal.pone.0039039. Epub 2012 Jun 19. PLoS One. 2012. PMID: 22723922 Free PMC article.
-
CD22-antagonists with nanomolar potency: the synergistic effect of hydrophobic groups at C-2 and C-9 of sialic acid scaffold.Bioorg Med Chem. 2011 Mar 15;19(6):1966-71. doi: 10.1016/j.bmc.2011.01.060. Epub 2011 Feb 2. Bioorg Med Chem. 2011. PMID: 21349726 Free PMC article.
-
The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans.Nat Rev Drug Discov. 2021 Mar;20(3):217-243. doi: 10.1038/s41573-020-00093-1. Epub 2021 Jan 18. Nat Rev Drug Discov. 2021. PMID: 33462432 Free PMC article. Review.
-
Catanionic Vesicles as a Facile Scaffold to Display Natural N-Glycan Ligands for Probing Multivalent Carbohydrate-Lectin Interactions.Bioconjug Chem. 2023 Feb 15;34(2):392-404. doi: 10.1021/acs.bioconjchem.2c00560. Epub 2023 Jan 15. Bioconjug Chem. 2023. PMID: 36642983 Free PMC article.
-
Glycoengineering in antigen-specific immunotherapies.Curr Opin Chem Biol. 2024 Aug;81:102503. doi: 10.1016/j.cbpa.2024.102503. Epub 2024 Jul 24. Curr Opin Chem Biol. 2024. PMID: 39053235 Free PMC article. Review.
References
-
- American Cancer Society: cancer facts and figures. American Cancer Society. 2008. www.cancer.org.
-
- Bello C, Sotomayor EM. Monoclonal antibodies for B-cell lymphomas: rituximab and beyond. Hematology Am Soc Hematol Educ Program. 2007;2007:233–242. - PubMed
-
- Castillo J, Winer E, Quesenberry P. Newer monoclonal antibodies for hematological malignancies. Exp Hematol. 2008 Jul;36(7):755–768. - PubMed
-
- Molina A. A decade of rituximab: improving survival outcomes in non-Hodgkin's lymphoma. Annu Rev Med. 2008;59:237–250. - PubMed
-
- Evans LS, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2003;362(9378):139–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

