EPAS1 Is Required for Spermatogenesis in the Postnatal Mouse Testis
- PMID: 20181618
- PMCID: PMC2874504
- DOI: 10.1095/biolreprod.109.079202
EPAS1 Is Required for Spermatogenesis in the Postnatal Mouse Testis
Abstract
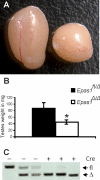
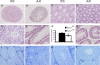
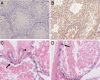
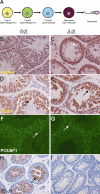
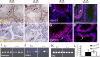
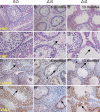
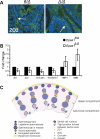
Spermatogenesis, a process involving the differentiation of spermatogonial stem cells into mature spermatozoa, takes place throughout masculine life. A complex system in the testis, including endocrine signaling, physical interactions between germ and somatic cells, spermatocyte meiosis, and timely release of spermatozoa, controls this cycle. We demonstrate herein that decreased O(2) levels and Epas1 activation are critical components of spermatogenesis. Postnatal Epas1 ablation leads to male infertility, with reduced testis size and weight. While immature spermatogonia and spermatocytes are present in Epas1(Delta/Delta) testes, spermatid and spermatozoan numbers are dramatically reduced. This is not due to germ cell-intrinsic defects. Rather, Epas(Delta/Delta) Sertoli cells exhibit decreased ability to form tight junctions, thereby disrupting the blood-testis barrier necessary for proper spermatogenesis. Reduced numbers of tight junction complexes are due to decreased expression of multiple genes encoding tight junction proteins, including TJP1 (ZO1), TJP2 (ZO2), and occludin. Furthermore, Epas1(Delta/Delta) testes exhibit disrupted basement membranes surrounding the seminiferous tubules, causing the premature release of incompletely differentiated germ cells. We conclude that low O(2) levels in the male gonad regulate germ cell homeostasis in this organ via EPAS1.
Figures


Similar articles
-
Hereditary defects in both germ cells and the blood-testis barrier system in as-mutant rats: evidence from spermatogonial transplantation and tracer-permeability analysis.Biol Reprod. 2002 Sep;67(3):880-8. doi: 10.1095/biolreprod.101.003061. Biol Reprod. 2002. PMID: 12193398
-
Annexin A2 is critical for blood-testis barrier integrity and spermatid disengagement in the mammalian testis.Biochim Biophys Acta Mol Cell Res. 2017 Mar;1864(3):527-545. doi: 10.1016/j.bbamcr.2016.12.012. Epub 2016 Dec 11. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 27974247
-
Connexin 43 reboots meiosis and reseals blood-testis barrier following toxicant-mediated aspermatogenesis and barrier disruption.FASEB J. 2016 Apr;30(4):1436-52. doi: 10.1096/fj.15-276527. Epub 2015 Dec 17. FASEB J. 2016. PMID: 26678449 Free PMC article.
-
Transport of germ cells across the seminiferous epithelium during spermatogenesis-the involvement of both actin- and microtubule-based cytoskeletons.Tissue Barriers. 2016 Nov 28;4(4):e1265042. doi: 10.1080/21688370.2016.1265042. eCollection 2016. Tissue Barriers. 2016. PMID: 28123928 Free PMC article. Review.
-
The Protective Role of L-Cysteine in the Regulation of Blood-Testis Barrier Functions-A Brief Review.Genes (Basel). 2024 Sep 12;15(9):1201. doi: 10.3390/genes15091201. Genes (Basel). 2024. PMID: 39336792 Free PMC article. Review.
Cited by
-
Hypoxia and spermatogenesis.Int Urol Nephrol. 2014 May;46(5):887-94. doi: 10.1007/s11255-013-0601-1. Epub 2013 Nov 22. Int Urol Nephrol. 2014. PMID: 24265038 Review.
-
Effect of low oxygen tension on transcriptional factor OCT4 and SOX2 expression in New Zealand rabbit bone marrow-derived mesenchymal stem cells.Vet World. 2020 Nov;13(11):2469-2476. doi: 10.14202/vetworld.2020.2469-2476. Epub 2020 Nov 18. Vet World. 2020. PMID: 33363343 Free PMC article.
-
Impact of the hypoxic microenvironment on spermatogonial stem cells in culture.Front Cell Dev Biol. 2024 Jan 18;11:1293068. doi: 10.3389/fcell.2023.1293068. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38304612 Free PMC article.
-
Regulation of cellular sterol homeostasis by the oxygen responsive noncoding RNA lincNORS.Nat Commun. 2020 Sep 21;11(1):4755. doi: 10.1038/s41467-020-18411-x. Nat Commun. 2020. PMID: 32958772 Free PMC article.
-
Follicle-stimulating hormone signaling in Sertoli cells: a licence to the early stages of spermatogenesis.Reprod Biol Endocrinol. 2022 Jul 2;20(1):97. doi: 10.1186/s12958-022-00971-w. Reprod Biol Endocrinol. 2022. PMID: 35780146 Free PMC article. Review.
References
-
- Vergouwen RP, Huiskamp R, Bas RJ, Roepers-Gajadien HL, Davids JA, de Rooij DG.Postnatal development of testicular cell populations in mice. J Reprod Fertil 1993; 99: 479–485. - PubMed
-
- Dym M, Fawcett DW.The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 1970; 3: 308–326. - PubMed
-
- Griswold MD.Interactions between germ cells and Sertoli cells in the testis. Biol Reprod 1995; 52: 211–216. - PubMed
-
- Russell LD, Peterson RN.Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol 1985; 94: 177–211. - PubMed
-
- Wenger RH, Katschinski DM.The hypoxic testis and post-meiotic expression of PAS domain proteins. Semin Cell Dev Biol 2005; 16: 547–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

