Role of ERalpha in the differential response of Stat5a loss in susceptibility to mammary preneoplasia and DMBA-induced carcinogenesis
- PMID: 20181624
- PMCID: PMC2878361
- DOI: 10.1093/carcin/bgq048
Role of ERalpha in the differential response of Stat5a loss in susceptibility to mammary preneoplasia and DMBA-induced carcinogenesis
Abstract
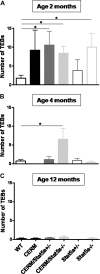
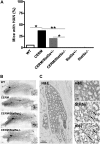
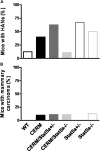
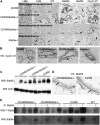
Deregulated estrogen signaling is evidently linked to breast cancer pathophysiology, although the role of signal transducer and activator of transcription (Stat)5a, integral to normal mammary gland development, is less clear. A mouse model of mammary epithelial cell-targeted deregulated estrogen receptor alpha (ERalpha) expression [conditional ERalpha in mammary epithelium (CERM)] was crossed with mice carrying a germ line deletion of Stat5a [Stat5a-/-] to investigate interactions between ERalpha and Stat5a in mammary tissue. CERM, CERM/Stat5a+/-, CERM/Stat5a-/-, Stat5a+/-, Stat5a-/- and wild-type (WT) mice were generated to test the roles of ERalpha and Stat5a on pubertal differentiation and cancer progression with and without exposure to the chemical carcinogen 7,12-dimethylbenz[a]anthracene (DMBA). Only CERM/Stat5a-/- mice demonstrated delayed pubertal terminal end bud differentiation. Without DMBA exposure, Stat5a loss abrogated ERalpha-initiated hyperplastic alveolar nodule (HAN) development and, similarly, Stat5a-/- mice did not develop HANs. However, although Stat5a loss still reduced ERalpha-initiated HAN prevalence following DMBA exposure, Stat5a loss without deregulated ERalpha was associated with an increased HAN prevalence compared with WT. Progression to ERalpha(+) and ERalpha(-) adenocarcinoma was found in all CERM-containing genotypes (CERM, CERM/Stat5a+/-, CERM/Stat5a-/-) and ERalpha(+) adenocarcinoma in the Stat5a-/- genotype. The mammary epithelial cell proliferative index was increased only in CERM mice independent of Stat5a loss. No differences in apoptotic indices were found. In summary, Stat5a cooperated with deregulated ERalpha in retarding pubertal mammary differentiation and contributed to ERalpha-initiated preneoplasia, but its loss did not prevent development of invasive cancer. Moreover, in the absence of deregulated ERalpha, Stat5a loss was associated with development of both HANs and invasive cancer following DMBA exposure.
Figures
References
-
- Speirs V, et al. New perspectives into the biological and clinical relevance of oestrogen receptors in the human breast. J. Pathol. 2007;211:499–506. - PubMed
-
- Allred DC, et al. Biological features of premalignant disease in the human breast. J. Mammary Gland Biol. Neoplasia. 2000;5:351–364. - PubMed
-
- Kuerer HM, et al. Ductal carcinoma in situ: state of the science and roadmap to advance the field. J. Clin. Oncol. 2009;27:279–288. - PubMed
-
- Medina D. Premalignant and malignant mammary lesions induced by MMTV and chemical carcinogens. J. Mammary Gland Biol. Neoplasia. 2008;13:271–277. - PubMed
-
- Allred DC, et al. The relevance of mouse models to understanding the development and progression of human breast cancer. J. Mammary Gland Biol. Neoplasia. 2008;13:279–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

