Acute regulation of renal Na+/H+ exchanger NHE3 by dopamine: role of protein phosphatase 2A
- PMID: 20181665
- PMCID: PMC2867406
- DOI: 10.1152/ajprenal.00708.2009
Acute regulation of renal Na+/H+ exchanger NHE3 by dopamine: role of protein phosphatase 2A
Abstract
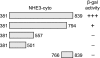
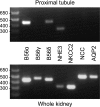
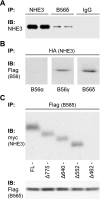
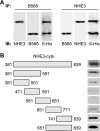
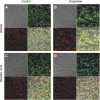
Nephrogenic dopamine is a potent natriuretic paracrine/autocrine hormone that is central for mammalian sodium homeostasis. In the renal proximal tubule, dopamine induces natriuresis partly via inhibition of the sodium/proton exchanger NHE3. The signal transduction pathways and mechanisms by which dopamine inhibits NHE3 are complex and incompletely understood. This manuscript describes the role of the serine/threonine protein phosphatase 2A (PP2A) in the regulation of NHE3 by dopamine. The PP2A regulatory subunit B56δ (coded by the Ppp2r5d gene) directly associates with more than one region of the carboxy-terminal hydrophilic putative cytoplasmic domain of NHE3 (NHE3-cyto), as demonstrated by yeast-two-hybrid, coimmunoprecipitation, blot overlay, and in vitro pull-down assays. Phosphorylated NHE3-cyto is a substrate for purified PP2A in an in vitro dephosphorylation reaction. In cultured renal cells, inhibition of PP2A by either okadaic acid or by overexpression of the simian virus 40 (SV40) small T antigen blocks the ability of dopamine to inhibit NHE3 activity and to reduce surface NHE3 protein. Dopamine-induced NHE3 redistribution is also blocked by okadaic acid ex vivo in rat kidney cortical slices. These studies demonstrate that PP2A is an integral and critical participant in the signal transduction pathway between dopamine receptor activation and NHE3 inhibition.
Figures
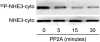
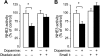

References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 215: 403–410, 1990 - PubMed
-
- Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int 48: 1206–1215, 1995 - PubMed
-
- Aperia A, Bertorello A, Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol Renal Fluid Electrolyte Physiol 252: F39–F45, 1987 - PubMed
-
- Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol 62: 621–647, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

