Role of the energy sensor AMP-activated protein kinase in renal physiology and disease
- PMID: 20181668
- PMCID: PMC2867412
- DOI: 10.1152/ajprenal.00005.2010
Role of the energy sensor AMP-activated protein kinase in renal physiology and disease
Abstract
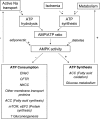
The ultrasensitive energy sensor AMP-activated protein kinase (AMPK) orchestrates the regulation of energy-generating and energy-consuming pathways. AMPK is highly expressed in the kidney where it is reported to be involved in a variety of physiological and pathological processes including ion transport, podocyte function, and diabetic renal hypertrophy. Sodium transport is the major energy-consuming process in the kidney, and AMPK has been proposed to contribute to the coupling of ion transport with cellular energy metabolism. Specifically, AMPK has been identified as a regulator of several ion transporters of significance in renal physiology, including the cystic fibrosis transmembrane conductance regulator (CFTR), the epithelial sodium channel (ENaC), the Na(+)-K(+)-2Cl(-) cotransporter (NKCC), and the vacuolar H(+)-ATPase (V-ATPase). Identified regulators of AMPK in the kidney include dietary salt, diabetes, adiponectin, and ischemia. Activation of AMPK in response to adiponectin is described in podocytes, where it reduces albuminuria, and in tubular cells, where it reduces glycogen accumulation. Reduced AMPK activity in the diabetic kidney is associated with renal accumulation of triglyceride and glycogen and the pathogenesis of diabetic renal hypertrophy. Acute renal ischemia causes a rapid and powerful activation of AMPK, but the functional significance of this observation remains unclear. Despite the recent advances, there remain significant gaps in the present understanding of both the upstream regulating pathways and the downstream substrates for AMPK in the kidney. A more complete understanding of the AMPK pathway in the kidney offers potential for improved therapies for several renal diseases including diabetic nephropathy, polycystic kidney disease, and ischemia-reperfusion injury.
Figures

References
-
- Almaca J, Kongsuphol P, Hieke B, Ousingsawat J, Viollet B, Schreiber R, Amaral MD, Kunzelmann K. AMPK controls epithelial Na+ channels through Nedd4–2 and causes an epithelial phenotype when mutated. Pflügers Arch 458: 713–721, 2009 - PubMed
-
- Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, Selbert MA, Edelman AM, Fremeau RT, Means AR. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase beta. J Biol Chem 273: 31880–31889, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

