The lack of an inherent membrane targeting signal is responsible for the failure of the matrix (M1) protein of influenza A virus to bud into virus-like particles
- PMID: 20181696
- PMCID: PMC2863757
- DOI: 10.1128/JVI.02306-09
The lack of an inherent membrane targeting signal is responsible for the failure of the matrix (M1) protein of influenza A virus to bud into virus-like particles
Abstract
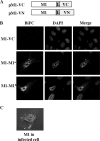
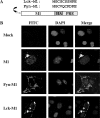
The matrix protein (M1) of influenza A virus is generally viewed as a key orchestrator in the release of influenza virions from the plasma membrane during infection. In contrast to this model, recent studies have indicated that influenza virus requires expression of the envelope proteins for budding of intracellular M1 into virus particles. Here we explored the mechanisms that control M1 budding. Similarly to previous studies, we found that M1 by itself fails to form virus-like-particles (VLPs). We further demonstrated that M1, in the absence of other viral proteins, was preferentially targeted to the nucleus/perinuclear region rather than to the plasma membrane, where influenza virions bud. Remarkably, we showed that a 10-residue membrane targeting peptide from either the Fyn or Lck oncoprotein appended to M1 at the N terminus redirected M1 to the plasma membrane and allowed M1 particle budding without additional viral envelope proteins. To further identify a functional link between plasma membrane targeting and VLP formation, we took advantage of the fact that M1 can interact with M2, unless the cytoplasmic tail is absent. Notably, native M2 but not mutant M2 effectively targeted M1 to the plasma membrane and produced extracellular M1 VLPs. Our results suggest that influenza virus M1 may not possess an inherent membrane targeting signal. Thus, the lack of efficient plasma membrane targeting is responsible for the failure of M1 in budding. This study highlights the fact that interactions of M1 with viral envelope proteins are essential to direct M1 to the plasma membrane for influenza virus particle release.
Figures



Similar articles
-
Involvement of an Arginine Triplet in M1 Matrix Protein Interaction with Membranes and in M1 Recruitment into Virus-Like Particles of the Influenza A(H1N1)pdm09 Virus.PLoS One. 2016 Nov 4;11(11):e0165421. doi: 10.1371/journal.pone.0165421. eCollection 2016. PLoS One. 2016. PMID: 27814373 Free PMC article.
-
Assembly and budding of influenza virus.Virus Res. 2004 Dec;106(2):147-65. doi: 10.1016/j.virusres.2004.08.012. Virus Res. 2004. PMID: 15567494 Free PMC article. Review.
-
Influenza A Virus M2 Protein Apical Targeting Is Required for Efficient Virus Replication.J Virol. 2018 Oct 29;92(22):e01425-18. doi: 10.1128/JVI.01425-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30158290 Free PMC article.
-
Lateral Organization of Influenza Virus Proteins in the Budozone Region of the Plasma Membrane.J Virol. 2017 Apr 13;91(9):e02104-16. doi: 10.1128/JVI.02104-16. Print 2017 May 1. J Virol. 2017. PMID: 28202765 Free PMC article.
-
Influenza virus morphogenesis and budding.Virus Res. 2009 Aug;143(2):147-61. doi: 10.1016/j.virusres.2009.05.010. Epub 2009 May 27. Virus Res. 2009. PMID: 19481124 Free PMC article. Review.
Cited by
-
A Glu-Glu-Tyr Sequence in the Cytoplasmic Tail of the M2 Protein Renders Influenza A Virus Susceptible to Restriction of the Hemagglutinin-M2 Association in Primary Human Macrophages.J Virol. 2022 Sep 28;96(18):e0071622. doi: 10.1128/jvi.00716-22. Epub 2022 Sep 13. J Virol. 2022. PMID: 36098511 Free PMC article.
-
Specific residues in the 2009 H1N1 swine-origin influenza matrix protein influence virion morphology and efficiency of viral spread in vitro.PLoS One. 2012;7(11):e50595. doi: 10.1371/journal.pone.0050595. Epub 2012 Nov 27. PLoS One. 2012. PMID: 23209789 Free PMC article.
-
A Single Vaccination of Chimeric Bivalent Virus-Like Particle Vaccine Confers Protection Against H9N2 and H3N2 Avian Influenza in Commercial Broilers and Allows a Strategy of Differentiating Infected from Vaccinated Animals.Front Immunol. 2022 Jul 8;13:902515. doi: 10.3389/fimmu.2022.902515. eCollection 2022. Front Immunol. 2022. PMID: 35874682 Free PMC article.
-
Involvement of an Arginine Triplet in M1 Matrix Protein Interaction with Membranes and in M1 Recruitment into Virus-Like Particles of the Influenza A(H1N1)pdm09 Virus.PLoS One. 2016 Nov 4;11(11):e0165421. doi: 10.1371/journal.pone.0165421. eCollection 2016. PLoS One. 2016. PMID: 27814373 Free PMC article.
-
The Matrix protein M1 from influenza C virus induces tubular membrane invaginations in an in vitro cell membrane model.Sci Rep. 2017 Jan 25;7:40801. doi: 10.1038/srep40801. Sci Rep. 2017. PMID: 28120862 Free PMC article.
References
-
- Bourmakina, S. V., and A. Garcia-Sastre. 2003. Reverse genetics studies on the filamentous morphology of influenza A virus. J. Gen. Virol. 84:517-527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

