Downregulation of Cdc2/CDK1 kinase activity induces the synthesis of noninfectious human papillomavirus type 31b virions in organotypic tissues exposed to benzo[a]pyrene
- PMID: 20181698
- PMCID: PMC2863740
- DOI: 10.1128/JVI.02431-09
Downregulation of Cdc2/CDK1 kinase activity induces the synthesis of noninfectious human papillomavirus type 31b virions in organotypic tissues exposed to benzo[a]pyrene
Abstract
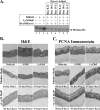
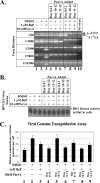
Epidemiological studies suggest that human papillomavirus (HPV)-infected women who smoke face an increased risk for developing cervical cancer. We have previously reported that exposure of HPV-positive organotypic cultures to benzo[a]pyrene (BaP), a major carcinogen in cigarette smoke, resulted in enhanced viral titers. Since BaP is known to deregulate multiple pathways of cellular proliferation, enhanced virion synthesis could result from carcinogen/host cell interaction. Here, we report that BaP-mediated upregulation of virus synthesis is correlated to an altered balance between cell cycle-specific cyclin-dependent kinase (CDK) activity profile compared with controls. Specifically, BaP treatment increased accumulation of hyperphosphorylated retinoblastoma protein (pRb) which coincided with increased cdc2/CDK1 kinase activity, but which further conflicted with the simultaneous upregulation of CDK inhibitors p16(INK4) and p27(KIP1), which normally mediate pRb hypophosphorylation. In contrast, p21(WAF1) and p53 levels remained unchanged. Under these conditions, CDK6 and CDK2 kinase activities were decreased, whereas CDK4 kinase activity remained unchanged. The addition of purvalanol A, a specific inhibitor of CDK1 kinase, to BaP-treated cultures, resulted in the production of noninfectious HPV type 31b (HPV31b) particles. In contrast, infectivity of control virus was unaffected by purvalanol A treatment. BaP targeting of CDK1 occurred independently of HPV status, since BaP treatment also increased CDK1 activity in tissues derived from primary keratinocytes. Our data indicate that HPV31b virions synthesized in the presence of BaP were dependent on BaP-mediated alteration in CDK1 kinase activity for maintaining their infectivity.
Figures



References
-
- Abelev, G. I. 2000. Differentiation mechanisms and malignancy. Biochemistry (Mosc.) 65:107-116. - PubMed
-
- Andrysik, Z., J. Vondracek, M. Machala, P. Krcmar, L. Svihalkova-Sindlerova, A. Kranz, C. Weiss, D. Faust, A. Kozubik, and C. Dietrich. 2007. The aryl hydrocarbon receptor-dependent deregulation of cell cycle control induced by polycyclic aromatic hydrocarbons in rat liver epithelial cells. Mutat. Res. 615:87-97. - PubMed
-
- Asselineau, D., and M. Prunieras. 1984. Reconstruction of ‘simplified’ skin: control of fabrication. Br. J. Dermatol. 111(Suppl. 27):219-222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

