T cells facilitate recovery from Venezuelan equine encephalitis virus-induced encephalomyelitis in the absence of antibody
- PMID: 20181704
- PMCID: PMC2863737
- DOI: 10.1128/JVI.02545-09
T cells facilitate recovery from Venezuelan equine encephalitis virus-induced encephalomyelitis in the absence of antibody
Abstract
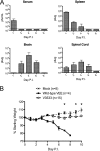
Venezuelan equine encephalitis virus (VEEV) is a mosquito-borne RNA virus of the genus Alphavirus that is responsible for a significant disease burden in Central and South America through sporadic outbreaks into human and equid populations. For humans, 2 to 4% of cases are associated with encephalitis, and there is an overall case mortality rate of approximately 1%. In mice, replication of the virus within neurons of the central nervous system (CNS) leads to paralyzing, invariably lethal encephalomyelitis. However, mice infected with certain attenuated mutants of the virus are able to control the infection within the CNS and recover. To better define what role T cell responses might be playing in this process, we infected B cell-deficient microMT mice with a VEEV mutant that induces mild, sublethal illness in immune competent mice. Infected microMT mice rapidly developed the clinical signs of severe paralyzing encephalomyelitis but were eventually able to control the infection and recover fully from clinical illness. Recovery in this system was T cell dependent and associated with a dramatic reduction in viral titers within the CNS, followed by viral persistence in the brain. Further comparison of the relative roles of T cell subpopulations within this system revealed that CD4(+) T cells were better producers of gamma interferon (IFN-gamma) than CD8(+) T cells and were more effective at controlling VEEV within the CNS. Overall, these results suggest that T cells, especially CD4(+) T cells, can successfully control VEEV infection within the CNS and facilitate recovery from a severe viral encephalomyelitis.
Figures
References
-
- Aronson, J. F., F. B. Grieder, N. L. Davis, P. C. Charles, T. Knott, K. Brown, and R. E. Johnston. 2000. A single-site mutant and revertants arising in vivo define early steps in the pathogenesis of Venezuelan equine encephalitis virus. Virology 270:111-123. - PubMed
-
- Bergmann, C. C., C. Ramakrishna, M. Kornacki, and S. A. Stohlman. 2001. Impaired T cell immunity in B cell-deficient mice following viral central nervous system infection. J. Immunol. 167:1575-1583. - PubMed
-
- Bernard, K. A., W. B. Klimstra, and R. E. Johnston. 2000. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology 276:93-103. - PubMed
-
- Bilzer, T., and L. Stitz. 1994. Immune-mediated brain atrophy. CD8+ T cells contribute to tissue destruction during borna disease. J. Immunol. 153:818-823. - PubMed
-
- Binder, G. K., and D. E. Griffin. 2001. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science 293:303-306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

