Identification of a dominant negative inhibitor of human zinc finger antiviral protein reveals a functional endogenous pool and critical homotypic interactions
- PMID: 20181706
- PMCID: PMC2863759
- DOI: 10.1128/JVI.02018-09
Identification of a dominant negative inhibitor of human zinc finger antiviral protein reveals a functional endogenous pool and critical homotypic interactions
Abstract
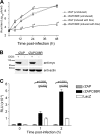
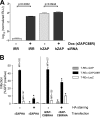
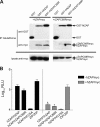
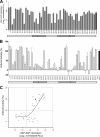
The zinc finger antiviral protein (ZAP) is a host factor with potent antiviral activity when overexpressed in cells. ZAP blocks replication of the prototype alphavirus Sindbis virus (SINV) at a step at or before translation of the incoming viral genome. The mechanism of ZAP anti-SINV activity and the determinants of its antiviral function, however, have not been defined. Here, we have identified a dominant negative inhibitor of human ZAP. Rat ZAP with a cysteine-to-arginine mutation at position 88 (rZAPC88R), previously reported as a nonfunctional form of ZAP, increases SINV growth in cells. These results led us to discover a previously undetectable pool of endogenous functional ZAP within human cells. Investigation of the mechanism of dominant negative inhibition, combined with a comprehensive mutational analysis of the antiviral factor, revealed that homotypic associations are required for ZAP function in limiting SINV propagation.
Figures
Similar articles
-
The zinc finger antiviral protein acts synergistically with an interferon-induced factor for maximal activity against alphaviruses.J Virol. 2007 Dec;81(24):13509-18. doi: 10.1128/JVI.00402-07. Epub 2007 Oct 10. J Virol. 2007. PMID: 17928353 Free PMC article.
-
Multiple interferon stimulated genes synergize with the zinc finger antiviral protein to mediate anti-alphavirus activity.PLoS One. 2012;7(5):e37398. doi: 10.1371/journal.pone.0037398. Epub 2012 May 16. PLoS One. 2012. PMID: 22615998 Free PMC article.
-
TRIM25 Enhances the Antiviral Action of Zinc-Finger Antiviral Protein (ZAP).PLoS Pathog. 2017 Jan 6;13(1):e1006145. doi: 10.1371/journal.ppat.1006145. eCollection 2017 Jan. PLoS Pathog. 2017. PMID: 28060952 Free PMC article.
-
Versatility of the Zinc-Finger Antiviral Protein (ZAP) As a Modulator of Viral Infections.Int J Biol Sci. 2024 Aug 26;20(12):4585-4600. doi: 10.7150/ijbs.98029. eCollection 2024. Int J Biol Sci. 2024. PMID: 39309436 Free PMC article. Review.
-
Antiviral Activity of Zinc Finger Antiviral Protein (ZAP) in Different Virus Families.Pathogens. 2023 Dec 17;12(12):1461. doi: 10.3390/pathogens12121461. Pathogens. 2023. PMID: 38133344 Free PMC article. Review.
Cited by
-
Nuclear matrix protein Matrin 3 is a regulator of ZAP-mediated retroviral restriction.Retrovirology. 2015 Jul 1;12:57. doi: 10.1186/s12977-015-0182-4. Retrovirology. 2015. PMID: 26129669 Free PMC article.
-
Structure of N-terminal domain of ZAP indicates how a zinc-finger protein recognizes complex RNA.Nat Struct Mol Biol. 2012 Mar 11;19(4):430-5. doi: 10.1038/nsmb.2243. Nat Struct Mol Biol. 2012. PMID: 22407013
-
S-farnesylation is essential for antiviral activity of the long ZAP isoform against RNA viruses with diverse replication strategies.PLoS Pathog. 2021 Oct 25;17(10):e1009726. doi: 10.1371/journal.ppat.1009726. eCollection 2021 Oct. PLoS Pathog. 2021. PMID: 34695163 Free PMC article.
-
Identification and characterization of the host protein DNAJC14 as a broadly active flavivirus replication modulator.PLoS Pathog. 2011 Jan 13;7(1):e1001255. doi: 10.1371/journal.ppat.1001255. PLoS Pathog. 2011. PMID: 21249176 Free PMC article.
-
Prenylome profiling reveals S-farnesylation is crucial for membrane targeting and antiviral activity of ZAP long-isoform.Proc Natl Acad Sci U S A. 2013 Jul 2;110(27):11085-90. doi: 10.1073/pnas.1302564110. Epub 2013 Jun 17. Proc Natl Acad Sci U S A. 2013. PMID: 23776219 Free PMC article.
References
-
- Aliperti, G., and M. J. Schlesinger. 1978. Evidence for an autoprotease activity of Sindbis virus capsid protein. Virology 90:366-369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

