Identification of a dominant negative inhibitor of human zinc finger antiviral protein reveals a functional endogenous pool and critical homotypic interactions
- PMID: 20181706
- PMCID: PMC2863759
- DOI: 10.1128/JVI.02018-09
Identification of a dominant negative inhibitor of human zinc finger antiviral protein reveals a functional endogenous pool and critical homotypic interactions
Abstract
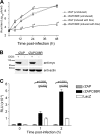
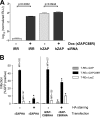
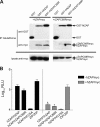
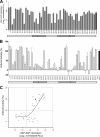
The zinc finger antiviral protein (ZAP) is a host factor with potent antiviral activity when overexpressed in cells. ZAP blocks replication of the prototype alphavirus Sindbis virus (SINV) at a step at or before translation of the incoming viral genome. The mechanism of ZAP anti-SINV activity and the determinants of its antiviral function, however, have not been defined. Here, we have identified a dominant negative inhibitor of human ZAP. Rat ZAP with a cysteine-to-arginine mutation at position 88 (rZAPC88R), previously reported as a nonfunctional form of ZAP, increases SINV growth in cells. These results led us to discover a previously undetectable pool of endogenous functional ZAP within human cells. Investigation of the mechanism of dominant negative inhibition, combined with a comprehensive mutational analysis of the antiviral factor, revealed that homotypic associations are required for ZAP function in limiting SINV propagation.
Figures
References
-
- Aliperti, G., and M. J. Schlesinger. 1978. Evidence for an autoprotease activity of Sindbis virus capsid protein. Virology 90:366-369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

