Pathogenic hantaviruses direct the adherence of quiescent platelets to infected endothelial cells
- PMID: 20181715
- PMCID: PMC2863738
- DOI: 10.1128/JVI.02405-09
Pathogenic hantaviruses direct the adherence of quiescent platelets to infected endothelial cells
Abstract
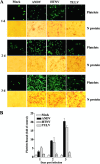
Hantavirus infections are noted for their ability to infect endothelial cells, cause acute thrombocytopenia, and trigger 2 vascular-permeability-based diseases. However, hantavirus infections are not lytic, and the mechanisms by which hantaviruses cause capillary permeability and thrombocytopenia are only partially understood. The role of beta(3) integrins in hemostasis and the inactivation of beta(3) integrin receptors by pathogenic hantaviruses suggest the involvement of hantaviruses in altered platelet and endothelial cell functions that regulate permeability. Here, we determined that pathogenic hantaviruses bind to quiescent platelets via a beta(3) integrin-dependent mechanism. This suggests that platelets may contribute to hantavirus dissemination within infected patients and provides a means by which hantavirus binding to beta(3) integrin receptors prevents platelet activation. The ability of hantaviruses to bind platelets further suggested that cell-associated hantaviruses might recruit platelets to the endothelial cell surface. Our findings indicate that Andes virus (ANDV)- or Hantaan virus (HTNV)-infected endothelial cells specifically direct the adherence of calcein-labeled platelets. In contrast, cells comparably infected with nonpathogenic Tula virus (TULV) failed to recruit platelets to the endothelial cell surface. Platelet adherence was dependent on endothelial cell beta(3) integrins and neutralized by the addition of the anti-beta(3) Fab fragment, c7E3, or specific ANDV- or HTNV-neutralizing antibodies. These findings indicate that pathogenic hantaviruses displayed on the surface of infected endothelial cells bind platelets and that a platelet layer covers the surface of infected endothelial cells. This fundamentally changes the appearance of endothelial cells and has the potential to alter cellular immune responses, platelet activation, and endothelial cell functions that affect vascular permeability. Hantavirus-directed platelet quiescence and recruitment to vast endothelial cell beds further suggests mechanisms by which hantaviruses may cause thrombocytopenia and induce hypoxia. These findings are fundamental to our understanding of pathogenic-hantavirus regulation of endothelial cell responses that contribute to vascular permeability.
Figures



References
-
- Aird, W. C. 2004. Endothelium as an organ system. Crit. Care Med. 32:S271-S279. - PubMed
-
- Baumgartner-Parzer, S. M., and W. K. Waldhausl. 2001. The endothelium as a metabolic and endocrine organ: its relation with insulin resistance. Exp. Clin. Endocrinol. Diabetes 109(Suppl. 2):S166-S179. - PubMed
-
- Berger, M. M., C. Hesse, C. Dehnert, H. Siedler, P. Kleinbongard, H. J. Bardenheuer, M. Kelm, P. Bartsch, and W. E. Haefeli. 2005. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med. 172:763-767. - PubMed
-
- Bombeli, T., B. R. Schwartz, and J. M. Harlan. 1998. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha. J. Exp. Med. 187:329-339. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

