Runx1 and Runx2 cooperate during sternal morphogenesis
- PMID: 20181744
- PMCID: PMC2835330
- DOI: 10.1242/dev.045005
Runx1 and Runx2 cooperate during sternal morphogenesis
Abstract
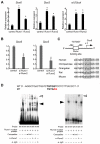
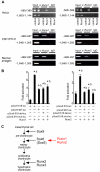
Chondrocyte differentiation is strictly regulated by various transcription factors, including Runx2 and Runx3; however, the physiological role of Runx1 in chondrocyte differentiation remains unknown. To examine the role of Runx1, we generated mesenchymal-cell-specific and chondrocyte-specific Runx1-deficient mice [Prx1 Runx1(f/f) mice and alpha1(II) Runx1(f/f) mice, respectively] to circumvent the embryonic lethality of Runx1-deficient mice. We then mated these mice with Runx2 mutant mice to obtain mesenchymal-cell-specific or chondrocyte-specific Runx1; Runx2 double-mutant mice [Prx1 DKO mice and alpha1(II) DKO mice, respectively]. Prx1 Runx1(f/f) mice displayed a delay in sternal development and Prx1 DKO mice completely lacked a sternum. By contrast, alpha1(II) Runx1(f/f) mice and alpha1(II) DKO mice did not show any abnormal sternal morphogenesis or chondrocyte differentiation. Notably, Runx1, Runx2 and the Prx1-Cre transgene were co-expressed specifically in the sternum, which explains the observation that the abnormalities were limited to the sternum. Histologically, mesenchymal cells condensed normally in the prospective sternum of Prx1 DKO mice; however, commitment to the chondrocyte lineage, which follows mesenchymal condensation, was significantly impaired. In situ hybridization analyses demonstrated that the expression of alpha1(II) collagen (Col2a1 - Mouse Genome Informatics), Sox5 and Sox6 in the prospective sternum of Prx1 DKO mice was severely attenuated, whereas Sox9 expression was unchanged. Molecular analyses revealed that Runx1 and Runx2 induce the expression of Sox5 and Sox6, which leads to the induction of alpha1(II) collagen expression via the direct regulation of promoter activity. Collectively, these results show that Runx1 and Runx2 cooperatively regulate sternal morphogenesis and the commitment of mesenchymal cells to become chondrocytes through the induction of Sox5 and Sox6.
Figures




References
-
- Bi W., Deng J. M., Zhang Z., Behringer R. R., de Crombrugghe B. (1999). Sox9 is required for cartilage formation. Nat. Genet. 22, 85-89 - PubMed
-
- Day T., Guo X., Garrett-Beal L., Yang Y. (2005). Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell 8, 739-750 - PubMed
-
- de Crombrugghe B., Lefebvre V., Nakashima K. (2001). Regulatory mechanisms in the pathways of cartilage and bone formation. Curr. Opin. Cell Biol. 13, 721-727 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

