Polysaccharide mimicry of the epitope of the broadly neutralizing anti-HIV antibody, 2G12, induces enhanced antibody responses to self oligomannose glycans
- PMID: 20181792
- PMCID: PMC2900896
- DOI: 10.1093/glycob/cwq020
Polysaccharide mimicry of the epitope of the broadly neutralizing anti-HIV antibody, 2G12, induces enhanced antibody responses to self oligomannose glycans
Abstract
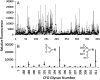
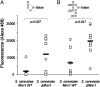
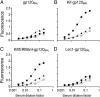
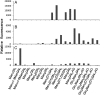
Immunologically, "self" carbohydrates protect the HIV-1 surface glycoprotein, gp120, from antibody recognition. However, one broadly neutralizing antibody, 2G12, neutralizes primary viral isolates by direct recognition of Manalpha1-->2Man motifs formed by the host-derived oligomannose glycans of the viral envelope. Immunogens, capable of eliciting antibodies of similar specificity to 2G12, are therefore candidates for HIV/AIDS vaccine development. In this context, it is known that the yeast mannan polysaccharides exhibit significant antigenic mimicry with the glycans of HIV-1. Here, we report that modulation of yeast polysaccharide biosynthesis directly controls the molecular specificity of cross-reactive antibodies to self oligomannose glycans. Saccharomyces cerevisiae mannans are typically terminated by alpha1-->3-linked mannoses that cap a Manalpha1-->2Man motif that otherwise closely resembles the part of the oligomannose epitope recognized by 2G12. Immunization with S. cerevisiae deficient for the alpha1-->3 mannosyltransferase gene (DeltaMnn1), but not with wild-type S. cerevisiae, reproducibly elicited antibodies to the self oligomannose glycans. Carbohydrate microarray analysis of DeltaMnn1 immune sera revealed fine carbohydrate specificity to Manalpha1-->2Man units, closely matching that of 2G12. These specificities were further corroborated by enzyme-linked immunosorbent assay with chemically defined glycoforms of gp120. These antibodies exhibited remarkable similarity in the carbohydrate specificity to 2G12 and displayed statistically significant, albeit extremely weak, neutralization of HIV-1 compared to control immune sera. These data confirm the Manalpha1-->2Man motif as the primary carbohydrate neutralization determinant of HIV-1 and show that the genetic modulation of microbial polysaccharides is a route towards immunogens capable of eliciting antibody responses to the glycans of HIV-1.
Figures


Similar articles
-
An engineered Saccharomyces cerevisiae strain binds the broadly neutralizing human immunodeficiency virus type 1 antibody 2G12 and elicits mannose-specific gp120-binding antibodies.J Virol. 2008 Jul;82(13):6447-57. doi: 10.1128/JVI.00412-08. Epub 2008 Apr 23. J Virol. 2008. PMID: 18434410 Free PMC article.
-
The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1-->2 mannose residues on the outer face of gp120.J Virol. 2002 Jul;76(14):7306-21. doi: 10.1128/jvi.76.14.7306-7321.2002. J Virol. 2002. PMID: 12072529 Free PMC article.
-
A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120.J Virol. 2008 Jul;82(13):6359-68. doi: 10.1128/JVI.00293-08. Epub 2008 Apr 23. J Virol. 2008. PMID: 18434393 Free PMC article.
-
Antibody responses to the HIV-1 envelope high mannose patch.Adv Immunol. 2019;143:11-73. doi: 10.1016/bs.ai.2019.08.002. Epub 2019 Sep 11. Adv Immunol. 2019. PMID: 31607367 Free PMC article. Review.
-
Glycans in Virus-Host Interactions: A Structural Perspective.Front Mol Biosci. 2021 Jun 7;8:666756. doi: 10.3389/fmolb.2021.666756. eCollection 2021. Front Mol Biosci. 2021. PMID: 34164431 Free PMC article. Review.
Cited by
-
Differential recognition of oligomannose isomers by glycan-binding proteins involved in innate and adaptive immunity.Sci Adv. 2021 Jun 9;7(24):eabf6834. doi: 10.1126/sciadv.abf6834. Print 2021 Jun. Sci Adv. 2021. PMID: 34108208 Free PMC article.
-
MALDI-MS/MS with traveling wave ion mobility for the structural analysis of N-linked glycans.J Am Soc Mass Spectrom. 2012 Nov;23(11):1955-66. doi: 10.1007/s13361-012-0425-8. Epub 2012 Sep 20. J Am Soc Mass Spectrom. 2012. PMID: 22993039
-
Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120.Nat Struct Mol Biol. 2013 Jul;20(7):796-803. doi: 10.1038/nsmb.2594. Epub 2013 May 26. Nat Struct Mol Biol. 2013. PMID: 23708606 Free PMC article.
-
Solution NMR analyses of the C-type carbohydrate recognition domain of DC-SIGNR protein reveal different binding modes for HIV-derived oligosaccharides and smaller glycan fragments.J Biol Chem. 2013 Aug 2;288(31):22745-57. doi: 10.1074/jbc.M113.458299. Epub 2013 Jun 20. J Biol Chem. 2013. PMID: 23788638 Free PMC article.
-
Antibodies expose multiple weaknesses in the glycan shield of HIV.Nat Struct Mol Biol. 2013 Jul;20(7):771-2. doi: 10.1038/nsmb.2627. Nat Struct Mol Biol. 2013. PMID: 23984441
References
-
- Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr D Biol Crystallogr. 2006;62:1243–1250. - PubMed
-
- Astronomo RD, Lee HK, Scanlan CN, Pantophlet R, Huang CY, Wilson IA, Blixt O, Dwek RA, Wong CH, Burton DR. A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120. J Virol. 2008;82:6359–6368. - PMC - PubMed
-
- Ballou CE. Isolation, characterization, and properties of Saccharomyces cerevisiae mnn mutants with nonconditional protein glycosylation defects. Methods Enzymol. 1990;185:440–470. - PubMed
-
- Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 2004;101:17033–17038. - PMC - PubMed
-
- Börnsen KO, Mohr MD, Widmer HM. Ion exchange and purification of carbohydrates on a Nafion(r) membrane as a new sample pretreatment for matrix- assisted laser desorption-ionization mass spectrometry. Rapid Commun Mass Spectrom. 1995;9:1031–1034.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

