Reduced prepubertal expression of progesterone receptor in the hypothalamus of female aromatase knockout mice
- PMID: 20181795
- PMCID: PMC2850240
- DOI: 10.1210/en.2009-1379
Reduced prepubertal expression of progesterone receptor in the hypothalamus of female aromatase knockout mice
Abstract
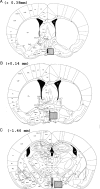
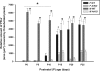
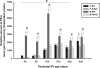
Previous research using alpha-fetoprotein knockout and aromatase knockout (ArKO) female mice suggested that the developing hypothalamic mechanisms that later control feminine sexual behavior are protected prenatally from estradiol, whereas shortly after birth, they may be stimulated by this same sex hormone. In the present study, we found that the amount of progesterone receptor immunoreactivity (PR-ir) in the anteroventral periventricular nucleus and medial part of the medial preoptic nucleus was significantly lower in ArKO female mice than in wild-type (WT) females at several prepubertal ages including postnatal d 15 (P15), P15, P20, and P25 but not neonatally at P0, P5, or P10. Likewise, PR-ir in the lateral subdivision of the ventromedial hypothalamic nucleus was significantly lower at P25 in ArKO vs. WT female mice but not at earlier postnatal ages. PR-ir was consistently higher in male than in female WT mice in the anteroventral periventricular nucleus and medial preoptic nucleus over P0-P10 and in the ventromedial hypothalamic nucleus over P0-P20. In these brain regions across these latter ages, PR-ir in male ArKO mice was significantly lower than in WT males and resembled the values seen in WT females, confirming previous reports that estradiol formed in the developing male hypothalamus from testicular testosterone is responsible for male-typical levels of neural PR expression. Thus, estradiol induces both female- and male-typical expression of PR postnatally in the mouse hypothalamus. Future experiments will determine whether this estradiol-induced PR expression contributes to either female- or male-typical brain and behavioral differentiation.
Figures


Similar articles
-
The two kisspeptin neuronal populations are differentially organized and activated by estradiol in mice.Endocrinology. 2013 Aug;154(8):2739-49. doi: 10.1210/en.2013-1120. Epub 2013 Jun 6. Endocrinology. 2013. PMID: 23744640
-
Hypothalamic expression of oestrogen receptor α and androgen receptor is sex-, age- and region-dependent in mice.J Neuroendocrinol. 2015 Apr;27(4):264-76. doi: 10.1111/jne.12258. J Neuroendocrinol. 2015. PMID: 25599767
-
Aromatase knockout mice show normal steroid-induced activation of gonadotrophin-releasing hormone neurones and luteinising hormone surges with a reduced population of kisspeptin neurones in the rostral hypothalamus.J Neuroendocrinol. 2012 Sep;24(9):1222-33. doi: 10.1111/j.1365-2826.2012.02334.x. J Neuroendocrinol. 2012. PMID: 22577852
-
The aromatase knockout (ArKO) mouse provides new evidence that estrogens are required for the development of the female brain.Ann N Y Acad Sci. 2003 Dec;1007:251-62. doi: 10.1196/annals.1286.024. Ann N Y Acad Sci. 2003. PMID: 14993058 Review.
-
Sex-steroid receptor mechanism related to neuronal aromatase and the stigmoid body.Horm Behav. 1994 Dec;28(4):545-55. doi: 10.1006/hbeh.1994.1053. Horm Behav. 1994. PMID: 7729824 Review.
Cited by
-
Aromatase promoter I.f is regulated by progesterone receptor in mouse hypothalamic neuronal cell lines.J Mol Endocrinol. 2011 Jul 18;47(1):69-80. doi: 10.1530/JME-10-0149. Print 2011 Aug. J Mol Endocrinol. 2011. PMID: 21628418 Free PMC article.
-
Female sexual behavior in mice is controlled by kisspeptin neurons.Nat Commun. 2018 Jan 26;9(1):400. doi: 10.1038/s41467-017-02797-2. Nat Commun. 2018. PMID: 29374161 Free PMC article.
-
Neural and Hormonal Basis of Opposite-Sex Preference by Chemosensory Signals.Int J Mol Sci. 2021 Aug 2;22(15):8311. doi: 10.3390/ijms22158311. Int J Mol Sci. 2021. PMID: 34361077 Free PMC article. Review.
-
Contribution of pheromones processed by the main olfactory system to mate recognition in female mammals.Front Neuroanat. 2012 Jun 5;6:20. doi: 10.3389/fnana.2012.00020. eCollection 2012. Front Neuroanat. 2012. PMID: 22679420 Free PMC article.
-
Evidence that sex chromosome genes affect sexual differentiation of female sexual behavior.Horm Behav. 2012 May;61(5):719-24. doi: 10.1016/j.yhbeh.2012.03.008. Epub 2012 Mar 30. Horm Behav. 2012. PMID: 22483977 Free PMC article.
References
-
- De Vries GJ, Simerly RB 2002 Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, eds. Hormones, brain and behavior. Amsterdam: Academic Press; 137–191
-
- Baum MJ, Carroll RS, Cherrv JA, Tobet SA 1990 Steroidal control of behavioural, neuroendocrine and brain sexual differentiation: studies in a carnivore, the ferret. J Neuroendocrinol 2:401–418 - PubMed
-
- McEwen BS, Plapinger L, Chaptal C, Gerlach J, Wallach G 1975 Role of fetoneonatal estrogen binding proteins in the associations of estrogen with neonatal brain cell nuclear receptors. Brain Res 96:400–406 - PubMed
-
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C 2006 Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci 9:220–226 - PubMed
-
- Toran-Allerand CD 1976 Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro: implications for sexual differentiation. Brain Res 106:407–412 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

