Proteomic analysis of V-ATPase-rich cells harvested from the kidney and epididymis by fluorescence-activated cell sorting
- PMID: 20181927
- PMCID: PMC2889637
- DOI: 10.1152/ajpcell.00552.2009
Proteomic analysis of V-ATPase-rich cells harvested from the kidney and epididymis by fluorescence-activated cell sorting
Abstract
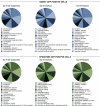
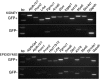
Proton-transporting cells are located in several tissues where they acidify the extracellular environment. These cells express the vacuolar H(+)-ATPase (V-ATPase) B1 subunit (ATP6V1B1) in their plasma membrane. We provide here a comprehensive catalog of the proteins that are expressed in these cells, after their isolation by enzymatic digestion and fluorescence-activated cell sorting (FACS) from transgenic B1-enhanced green fluorescent protein (EGFP) mice. In these mice, type A and B intercalated cells and connecting segment cells of the kidney, and narrow and clear cells of the epididymis, which all express ATP6V1B1, also express EGFP, while all other cell types are negative. The proteome of renal and epididymal EGFP-positive (EGFP(+)) cells was identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and compared with their respective EGFP-negative (EGFP(-)) cell populations. A total of 2,297 and 1,564 proteins were detected in EGFP(+) cells from the kidney and epididymis, respectively. Out of these proteins, 202 and 178 were enriched by a factor greater than 1.5 in EGFP(+) cells compared with EGFP(-) cells, in the kidney and epididymis respectively, and included subunits of the V-ATPase (B1, a4, and A). In addition, several proteins involved in intracellular trafficking, signaling, and cytoskeletal dynamics were identified. A novel common protein that was enriched in renal and epididymal EGFP(+) cells is the progesterone receptor, which might be a potential candidate for the regulation of V-ATPase-dependent proton transport. These proteomic databases provide a framework for comprehensive future analysis of the common and distinct functions of V-ATPase-B1-expressing cells in the kidney and epididymis.
Figures


Comment in
-
Proteomics reveal the breadth and limits of model systems inferences. Focus on "proteomic analysis of V-ATPase-rich cells harvested from the kidney and epididymis by fluorescence-activated cell sorting".Am J Physiol Cell Physiol. 2010 Jun;298(6):C1303-4. doi: 10.1152/ajpcell.00091.2010. Epub 2010 Mar 17. Am J Physiol Cell Physiol. 2010. PMID: 20237146 Free PMC article. No abstract available.
References
-
- Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+-ATPase (V-ATPase) recycling. J Biol Chem 280: 8452–8463, 2005 - PubMed
-
- Beyenbach KW, Wieczorek H. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol 209: 577–589, 2006 - PubMed
-
- Bielak-Zmijewska A, Kolano A, Szczepanska K, Maleszewski M, Borsuk E. Cdc42 protein acts upstream of IQGAP1 and regulates cytokinesis in mouse oocytes and embryos. Dev Biol 322: 21–32, 2008 - PubMed
-
- Blanchard PG, Luu-The V. Differential androgen and estrogen substrates specificity in the mouse and primates type 12 17beta-hydroxysteroid dehydrogenase. J Endocrinol 194: 449–455, 2007 - PubMed
-
- Boros S, Bindels RJ, Hoenderop JG. Active Ca(2+) reabsorption in the connecting tubule. Pflügers Arch 458: 99–109, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

