p38 MAP kinase is necessary for melanoma-mediated regulation of VE-cadherin disassembly
- PMID: 20181932
- PMCID: PMC2867383
- DOI: 10.1152/ajpcell.00242.2009
p38 MAP kinase is necessary for melanoma-mediated regulation of VE-cadherin disassembly
Abstract
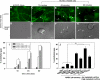
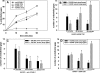
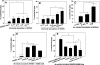
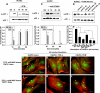
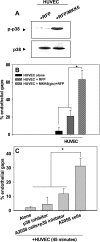
Vascular endothelial (VE)-cadherin is localized to the endothelial borders and the adherens junctions, which are regulated by changes in mitogen-activated protein (MAP) kinases, GTPases, and intracellular calcium. We previously showed that melanoma cells induce VE-cadherin disassembly through contact with human umbilical vein endothelial cells in coculture. However, the exact mechanism by which melanoma cells signal endothelial cells to induce VE-cadherin junction disassembly is not well understood. In this study, VE-cadherin junction disassembly was further examined under fluorescence microscopy. We found that melanoma-induced VE-cadherin junction disassembly and upregulation of p38 MAP kinase in endothelial cells is regulated by both soluble factors from melanomas, particularly interleukin (IL)-8, IL-6, and IL-1beta, and through vascular cell adhesion molecule-1. Neutralizing melanoma-secreted soluble factors reduced endothelial gap formation. Endothelial cells transfected with MAP kinase kinase 6, a direct activator of p38 MAP kinase, increased VE-cadherin-mediated gap formation, facilitating melanoma transendothelial migration. In contrast, endothelial cells transfected with small-interfering RNA against p38 MAP kinase expression largely prevented melanoma transendothelial migration in Boyden chamber experiments. These findings indicate that p38 MAP kinase proteins regulate VE-cadherin junction disassembly, facilitating melanoma migration across endothelial cells.
Figures
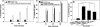

Similar articles
-
Mutant B-Raf(V600E) Promotes Melanoma Paracellular Transmigration by Inducing Thrombin-mediated Endothelial Junction Breakdown.J Biol Chem. 2016 Jan 29;291(5):2087-106. doi: 10.1074/jbc.M115.696419. Epub 2015 Oct 26. J Biol Chem. 2016. PMID: 26504080 Free PMC article.
-
VE-Cadherin Disassembly and Cell Contractility in the Endothelium are Necessary for Barrier Disruption Induced by Tumor Cells.Sci Rep. 2017 Apr 10;7:45835. doi: 10.1038/srep45835. Sci Rep. 2017. PMID: 28393886 Free PMC article.
-
Actinomyosin contraction, phosphorylation of VE-cadherin, and actin remodeling enable melanoma-induced endothelial cell-cell junction disassembly.PLoS One. 2014 Sep 16;9(9):e108092. doi: 10.1371/journal.pone.0108092. eCollection 2014. PLoS One. 2014. PMID: 25225982 Free PMC article.
-
Dynamic Regulation of Vascular Permeability by Vascular Endothelial Cadherin-Mediated Endothelial Cell-Cell Junctions.J Nippon Med Sch. 2017;84(4):148-159. doi: 10.1272/jnms.84.148. J Nippon Med Sch. 2017. PMID: 28978894 Review.
-
VE-cadherin: at the front, center, and sides of endothelial cell organization and function.Curr Opin Cell Biol. 2010 Oct;22(5):651-8. doi: 10.1016/j.ceb.2010.07.006. Epub 2010 Aug 11. Curr Opin Cell Biol. 2010. PMID: 20708398 Free PMC article. Review.
Cited by
-
Caspase-8 modulates physiological and pathological angiogenesis during retina development.J Clin Invest. 2019 Dec 2;129(12):5092-5107. doi: 10.1172/JCI122767. J Clin Invest. 2019. PMID: 31454332 Free PMC article.
-
Thrombomodulin blocks calcineurin inhibitor-induced vascular permeability via inhibition of Src/VE-cadherin axis.Bone Marrow Transplant. 2017 Feb;52(2):245-251. doi: 10.1038/bmt.2016.241. Epub 2016 Sep 19. Bone Marrow Transplant. 2017. PMID: 27643869
-
Mutant B-Raf(V600E) Promotes Melanoma Paracellular Transmigration by Inducing Thrombin-mediated Endothelial Junction Breakdown.J Biol Chem. 2016 Jan 29;291(5):2087-106. doi: 10.1074/jbc.M115.696419. Epub 2015 Oct 26. J Biol Chem. 2016. PMID: 26504080 Free PMC article.
-
Heat shock protein 27 activity is linked to endothelial barrier recovery after proinflammatory GPCR-induced disruption.Sci Signal. 2021 Aug 31;14(698):eabc1044. doi: 10.1126/scisignal.abc1044. Epub 2021 Aug 31. Sci Signal. 2021. PMID: 34516752 Free PMC article.
-
Prolyl Hydroxylase Domain-2 Protein Regulates Lipopolysaccharide-Induced Vascular Inflammation.Am J Pathol. 2019 Jan;189(1):200-213. doi: 10.1016/j.ajpath.2018.09.012. Epub 2018 Oct 17. Am J Pathol. 2019. PMID: 30339838 Free PMC article.
References
-
- Dejana E, Bazzoni G, Lampugnani MG. Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp Cell Res 252: 13–19, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

