Cockayne syndrome group B protein promotes mitochondrial DNA stability by supporting the DNA repair association with the mitochondrial membrane
- PMID: 20181933
- PMCID: PMC2887265
- DOI: 10.1096/fj.09-147991
Cockayne syndrome group B protein promotes mitochondrial DNA stability by supporting the DNA repair association with the mitochondrial membrane
Abstract
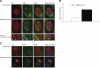
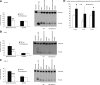
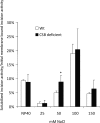
Cockayne syndrome (CS) is a human premature aging disorder associated with severe developmental deficiencies and neurodegeneration, and phenotypically it resembles some mitochondrial DNA (mtDNA) diseases. Most patients belong to complementation group B, and the CS group B (CSB) protein plays a role in genomic maintenance and transcriptome regulation. By immunocytochemistry, mitochondrial fractionation, and Western blotting, we demonstrate that CSB localizes to mitochondria in different types of cells, with increased mitochondrial distribution following menadione-induced oxidative stress. Moreover, our results suggest that CSB plays a significant role in mitochondrial base excision repair (BER) regulation. In particular, we find reduced 8-oxo-guanine, uracil, and 5-hydroxy-uracil BER incision activities in CSB-deficient cells compared to wild-type cells. This deficiency correlates with deficient association of the BER activities with the mitochondrial inner membrane, suggesting that CSB may participate in the anchoring of the DNA repair complex. Increased mutation frequency in mtDNA of CSB-deficient cells demonstrates functional significance of the presence of CSB in the mitochondria. The results in total suggest that CSB plays a direct role in mitochondrial BER by helping recruit, stabilize, and/or retain BER proteins in repair complexes associated with the inner mitochondrial membrane, perhaps providing a novel basis for understanding the complex phenotype of this debilitating disorder.
Figures


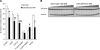


References
-
- Balajee A S, Proietti De Santis L, Brosh R M, Jr, Selzer R, Bohr V A. Role of the ATPase domain of the Cockayne syndrome group B protein in UV induced apoptosis. Oncogene. 2000;19:477–489. - PubMed
-
- Lehmann A R. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie (Paris) 2003;85:1101–1111. - PubMed
-
- Stevnsner T, Nyaga S, de Souza-Pinto N C, van der Horst G T, Gorgels T G, Hogue B A, Thorslund T, Bohr V A. Mitochondrial repair of 8-oxoguanine is deficient in Cockayne syndrome group B. Oncogene. 2002;21:8675–8682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

