CCL8/MCP-2 is a target for mir-146a in HIV-1-infected human microglial cells
- PMID: 20181935
- PMCID: PMC2887261
- DOI: 10.1096/fj.09-143503
CCL8/MCP-2 is a target for mir-146a in HIV-1-infected human microglial cells
Abstract
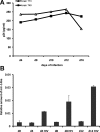
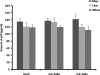
MicroRNA-mediated regulation of gene expression appears to be involved in a variety of cellular processes, including development, differentiation, proliferation, and apoptosis. Mir-146a is thought to be involved in the regulation of the innate immune response, and its expression is increased in tissues associated with chronic inflammation. Among the predicted gene targets for mir-146a, the chemokine CCL8/MCP-2 is a ligand for the CCR5 chemokine receptor and a potent inhibitor of CD4/CCR5-mediated HIV-1 entry and replication. In the present study, we have analyzed changes in the expression of mir-146a in primary human fetal microglial cells upon infection with HIV-1 and found increased expression of mir-146a. We further show that CCL8/MCP-2 is a target for mir-146a in HIV-1 infected microglia, as overexpression of mir-146a prevented HIV-induced secretion of MCP-2 chemokine. The clinical relevance of our findings was evaluated in HIV-encephalitis (HIVE) brain samples in which decreased levels of MCP-2 and increased levels of mir-146a were observed, suggesting a role for mir-146a in the maintenance of HIV-mediated chronic inflammation of the brain.
Figures
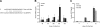



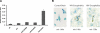
Similar articles
-
MicroRNA-146a-5p attenuates visceral hypersensitivity through targeting chemokine CCL8 in the spinal cord in a mouse model of colitis.Brain Res Bull. 2018 May;139:235-242. doi: 10.1016/j.brainresbull.2018.03.007. Epub 2018 Mar 14. Brain Res Bull. 2018. PMID: 29550454
-
miR-146a suppresses cellular immune response during Japanese encephalitis virus JaOArS982 strain infection in human microglial cells.J Neuroinflammation. 2015 Feb 18;12:30. doi: 10.1186/s12974-015-0249-0. J Neuroinflammation. 2015. PMID: 25889446 Free PMC article.
-
MiR-155 induction in microglial cells suppresses Japanese encephalitis virus replication and negatively modulates innate immune responses.J Neuroinflammation. 2014 May 29;11:97. doi: 10.1186/1742-2094-11-97. J Neuroinflammation. 2014. PMID: 24885259 Free PMC article.
-
The role of miR-146a in viral infection.IUBMB Life. 2020 Mar;72(3):343-360. doi: 10.1002/iub.2222. Epub 2019 Dec 30. IUBMB Life. 2020. PMID: 31889417 Review.
-
Long noncoding RNA SNHG16 targets miR-146a-5p/CCL5 to regulate LPS-induced WI-38 cell apoptosis and inflammation in acute pneumonia.Life Sci. 2019 Jul 1;228:189-197. doi: 10.1016/j.lfs.2019.05.008. Epub 2019 May 7. Life Sci. 2019. PMID: 31071307 Review.
Cited by
-
Regulatory role of microRNAs in virus-mediated inflammation.J Inflamm (Lond). 2024 Nov 4;21(1):43. doi: 10.1186/s12950-024-00417-7. J Inflamm (Lond). 2024. PMID: 39497125 Free PMC article. Review.
-
Roles of microglia in brain development, tissue maintenance and repair.Brain. 2015 May;138(Pt 5):1138-59. doi: 10.1093/brain/awv066. Epub 2015 Mar 29. Brain. 2015. PMID: 25823474 Free PMC article. Review.
-
Involvement of TLR2-MyD88 in abnormal expression of miR-146a in peripheral blood monocytes of patients with chronic hepatitis C.J Huazhong Univ Sci Technolog Med Sci. 2015 Apr;35(2):219-224. doi: 10.1007/s11596-015-1414-5. Epub 2015 Apr 16. J Huazhong Univ Sci Technolog Med Sci. 2015. PMID: 25877355
-
Divergent Cytokine and Chemokine Responses at Early Acute Simian Immunodeficiency Virus Infection Correlated with Virus Replication and CD4 T Cell Loss in a Rhesus Macaque Model.Vaccines (Basel). 2023 Jan 25;11(2):264. doi: 10.3390/vaccines11020264. Vaccines (Basel). 2023. PMID: 36851142 Free PMC article.
-
Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations.Acta Neuropathol. 2018 Apr;135(4):529-550. doi: 10.1007/s00401-017-1803-x. Epub 2018 Jan 4. Acta Neuropathol. 2018. PMID: 29302779 Free PMC article.
References
-
- Bartel D P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol. 2008;18:131–140. - PubMed
-
- Bagasra O, Prilliman K R. RNA interference: the molecular immune system. J Mol Histol. 2004;35:545–553. - PubMed
-
- Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

