Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase
- PMID: 20181949
- PMCID: PMC2857065
- DOI: 10.1074/jbc.M109.088864
Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase
Abstract
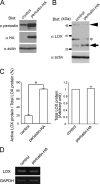
Intra- and intermolecular covalent cross-linking between collagen fibrils, catalyzed by lysyl oxidase (LOX), determines the mechanical properties of connective tissues; however, mechanisms that regulate the collagen cross-linking according to tissue specificity are not well understood. Here we show that periostin, a secretory protein in the dense connective tissues, promotes the activation of LOX. Previous studies showed that periostin null mice exhibit reduced collagen cross-linking in their femurs, periosteum, infarcted myocardium, and tendons. Presently, we showed that active LOX protein, formed by cleavage of its propeptide by bone morphogenetic protein-1 (BMP-1), was decreased in calvarial osteoblast cells derived from periostin null mice. Overexpression of periostin promoted the proteolytic cleavage of the propeptide, which increased the amount of active LOX protein. The results of co-immunoprecipitation and solid phase binding assays revealed that periostin interacted with BMP-1. Furthermore, this interaction probably resulted in enhanced deposition of BMP-1 on the extracellular matrix, suggesting that this enhanced deposition would lead to cleavage of the propeptide of LOX. Thus, we demonstrated that periostin supported BMP-1-mediated proteolytic activation of LOX on the extracellular matrix, which promoted collagen cross-linking.
Figures



References
-
- Canty E. G., Kadler K. E. (2005) J. Cell Sci. 118, 1341–1353 - PubMed
-
- Banse X., Sims T. J., Bailey A. J. (2002) J. Bone Miner. Res. 17, 1621–1628 - PubMed
-
- Saito M., Fujii K., Mori Y., Marumo K. (2006) Osteoporos. Int. 17, 1514–1523 - PubMed
-
- Seeman E., Delmas P. D. (2006) N. Engl. J. Med. 354, 2250–2261 - PubMed
-
- Açil Y., Vetter U., Brenner R., Müller P. K., Brinckmann J. (1995) J. Am. Acad. Dermatol. 33, 522–524 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

