SENP3-mediated de-conjugation of SUMO2/3 from promyelocytic leukemia is correlated with accelerated cell proliferation under mild oxidative stress
- PMID: 20181954
- PMCID: PMC2857110
- DOI: 10.1074/jbc.M109.071431
SENP3-mediated de-conjugation of SUMO2/3 from promyelocytic leukemia is correlated with accelerated cell proliferation under mild oxidative stress
Abstract
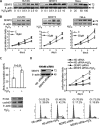
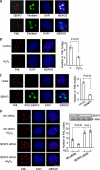
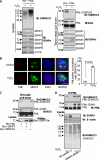
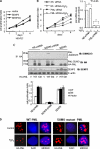
Small ubiquitin-like modifier (SUMO) 2/3 is known to conjugate to substrates in response to a variety of cellular stresses. However, whether and how SUMO2/3-specific proteases are involved in de-conjugation under cell stress is unclear. Here, we show that low doses of hydrogen peroxide (H(2)O(2)) induce an increase of the SENP3 protein, which removes SUMO2/3 from promyelocytic leukemia (PML). Low dose H(2)O(2) causes SENP3 to co-localize with PML bodies and reduces the number of PML bodies in a SENP3-dependent manner. Furthermore, de-conjugation of SUMO2/3 from PML is responsible for the accelerated cell proliferation caused by low dose H(2)O(2). Knocking down PML promotes basal cell proliferation as expected. This can be reversed by reconstitution with wild-type PML but not its mutant lacking SUMOylation, indicating that only the SUMOylated PML can play an inhibitory role for cell proliferation. Thus, SENP3 appears to be a key mediator in mild oxidative stress-induced cell proliferation via regulation of the SUMOylation status of PML. Furthermore, SENP3 is over-accumulated in a variety of primary human cancers including colon adenocarcinoma in which PML is hypo-SUMOylated. These results reveal an important role of SENP3 and the SUMOylation status of PML in the regulation of cell proliferation under oxidative stress.
Figures
Similar articles
-
SENP3 regulates the global protein turnover and the Sp1 level via antagonizing SUMO2/3-targeted ubiquitination and degradation.Protein Cell. 2016 Jan;7(1):63-77. doi: 10.1007/s13238-015-0216-7. Epub 2015 Oct 28. Protein Cell. 2016. PMID: 26511642 Free PMC article.
-
Solubility shift and SUMOylaltion of promyelocytic leukemia (PML) protein in response to arsenic(III) and fate of the SUMOylated PML.Toxicol Appl Pharmacol. 2015 Sep 15;287(3):191-201. doi: 10.1016/j.taap.2015.05.018. Epub 2015 Jun 3. Toxicol Appl Pharmacol. 2015. PMID: 26049103
-
Inhibiting ubiquitination causes an accumulation of SUMOylated newly synthesized nuclear proteins at PML bodies.J Biol Chem. 2019 Oct 18;294(42):15218-15234. doi: 10.1074/jbc.RA119.009147. Epub 2019 Jul 8. J Biol Chem. 2019. PMID: 31285264 Free PMC article.
-
A manually curated network of the PML nuclear body interactome reveals an important role for PML-NBs in SUMOylation dynamics.Int J Biol Sci. 2010 Jan 12;6(1):51-67. doi: 10.7150/ijbs.6.51. Int J Biol Sci. 2010. PMID: 20087442 Free PMC article. Review.
-
Unravelling the molecular interplay: SUMOylation, PML nuclear bodies and vascular cell activity in health and disease.Cell Signal. 2024 Jul;119:111156. doi: 10.1016/j.cellsig.2024.111156. Epub 2024 Apr 2. Cell Signal. 2024. PMID: 38574938 Review.
Cited by
-
SUMOylation and SENP3 regulate STAT3 activation in head and neck cancer.Oncogene. 2016 Nov 10;35(45):5826-5838. doi: 10.1038/onc.2016.124. Epub 2016 May 16. Oncogene. 2016. PMID: 27181202 Free PMC article.
-
SUMO-Specific Protease 2 (SENP2) Is an Important Regulator of Fatty Acid Metabolism in Skeletal Muscle.Diabetes. 2015 Jul;64(7):2420-31. doi: 10.2337/db15-0115. Epub 2015 Mar 17. Diabetes. 2015. PMID: 25784542 Free PMC article.
-
SENP3-mediated host defense response contains HBV replication and restores protein synthesis.PLoS One. 2019 Jan 14;14(1):e0209179. doi: 10.1371/journal.pone.0209179. eCollection 2019. PLoS One. 2019. PMID: 30640896 Free PMC article.
-
Small ubiquitin-like modifier 1-3 conjugation [corrected] is activated in human astrocytic brain tumors and is required for glioblastoma cell survival.Cancer Sci. 2013 Jan;104(1):70-7. doi: 10.1111/cas.12047. Epub 2012 Nov 28. Cancer Sci. 2013. PMID: 23078246 Free PMC article.
-
SUMO Losing Balance: SUMO Proteases Disrupt SUMO Homeostasis to Facilitate Cancer Development and Progression.Genes Cancer. 2010 Jul;1(7):748-752. doi: 10.1177/1947601910382555. Genes Cancer. 2010. PMID: 21152235 Free PMC article.
References
-
- Yeh E. T., Gong L., Kamitani T. (2000) Gene 248, 1–14 - PubMed
-
- Saitoh H., Hinchey J. (2000) J. Biol. Chem. 275, 6252–6258 - PubMed
-
- Manza L. L., Codreanu S. G., Stamer S. L., Smith D. L., Wells K. S., Roberts R. L., Liebler D. C. (2004) Chem. Res. Toxicol. 17, 1706–1715 - PubMed
-
- Li T., Santockyte R., Shen R. F., Tekle E., Wang G., Yang D. C., Chock P. B. (2006) J. Biol. Chem. 281, 36221–36227 - PubMed
-
- Dorval V., Fraser P. E. (2007) Biochim. Biophys. Acta 1773, 694–706 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

