Timing of initiation of antiretroviral drugs during tuberculosis therapy
- PMID: 20181971
- PMCID: PMC3076221
- DOI: 10.1056/NEJMoa0905848
Timing of initiation of antiretroviral drugs during tuberculosis therapy
Abstract
Background: The rates of death are high among patients with coinfection with tuberculosis and the human immunodeficiency virus (HIV). The optimal timing for the initiation of antiretroviral therapy in relation to tuberculosis therapy remains controversial.
Methods: In an open-label, randomized, controlled trial in Durban, South Africa, we assigned 642 patients with both tuberculosis and HIV infection to start antiretroviral therapy either during tuberculosis therapy (in two integrated-therapy groups) or after the completion of such treatment (in one sequential-therapy group). The diagnosis of tuberculosis was based on a positive sputum smear for acid-fast bacilli. Only patients with HIV infection and a CD4+ cell count of less than 500 per cubic millimeter were included. All patients received standard tuberculosis therapy, prophylaxis with trimethoprim-sulfamethoxazole, and a once-daily antiretroviral regimen of didanosine, lamivudine, and efavirenz. The primary end point was death from any cause.
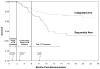
Results: This analysis compares data from the sequential-therapy group and the combined integrated-therapy groups up to September 1, 2008, when the data and safety monitoring committee recommended that all patients receive integrated antiretroviral therapy. There was a reduction in the rate of death among the 429 patients in the combined integrated-therapy groups (5.4 deaths per 100 person-years, or 25 deaths), as compared with the 213 patients in the sequential-therapy group (12.1 per 100 person-years, or 27 deaths); a relative reduction of 56% (hazard ratio in the combined integrated-therapy groups, 0.44; 95% confidence interval, 0.25 to 0.79; P=0.003). Mortality was lower in the combined integrated-therapy groups in all CD4+ count strata. Rates of adverse events during follow-up were similar in the two study groups.
Conclusions: The initiation of antiretroviral therapy during tuberculosis therapy significantly improved survival and provides further impetus for the integration of tuberculosis and HIV services. (ClinicalTrials.gov number, NCT00398996.)
2010 Massachusetts Medical Society
Figures
Comment in
-
Timing of antiretroviral drugs during tuberculosis therapy.N Engl J Med. 2010 Jun 3;362(22):2137; author reply 2138-9. doi: 10.1056/NEJMc1003767. N Engl J Med. 2010. PMID: 20519688 No abstract available.
-
Timing of antiretroviral drugs during tuberculosis therapy.N Engl J Med. 2010 Jun 3;362(22):2138; author reply 2138-9. N Engl J Med. 2010. PMID: 20527060 No abstract available.
-
Timing of antiretroviral drugs during tuberculosis therapy.N Engl J Med. 2010 Jun 3;362(22):2137-8; author reply 2138-9. N Engl J Med. 2010. PMID: 20527080 No abstract available.
References
-
- UNAIDS. 2008 Report on the global AIDS Epidemic Update. Geneva: Joint United Nations Programme on HIV/AIDS; [(Accessed 31 October 2008)]. 2008. http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_....
-
- World Health Organisation. Global Tuberculosis Control: Surveillance, planning, financing. Geneva, Switzerland: World Health Organisation; 2008.
-
- Abdool Karim SS. Durban 2000 to Toronto 2006: The evolving challenges in implementing AIDS treatment in Africa. AIDS (London, England) 2006;20:N7–N9. - PubMed
-
- Churchyard GJ, Kleinschmidt I, Corbett EL, Mulder D, Smit J, de Kock KM. Factors associated with an increased case-fatality rate in HIV-infected and non-infected South African gold miners with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4:705–12. - PubMed
-
- Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS (London, England) 2001;15:143–52. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials