Cancer vaccine by fusions of dendritic and cancer cells
- PMID: 20182533
- PMCID: PMC2825547
- DOI: 10.1155/2009/657369
Cancer vaccine by fusions of dendritic and cancer cells
Abstract
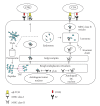
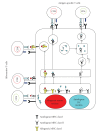
Dendritic cells (DCs) are potent antigen-presenting cells and play a central role in the initiation and regulation of primary immune responses. Therefore, their use for the active immunotherapy against cancers has been studied with considerable interest. The fusion of DCs with whole tumor cells represents in many ways an ideal approach to deliver, process, and subsequently present a broad array of tumor-associated antigens, including those yet to be unidentified, in the context of DCs-derived costimulatory molecules. DCs/tumor fusion vaccine stimulates potent antitumor immunity in the animal tumor models. In the human studies, T cells stimulated by DC/tumor fusion cells are effective in lysis of tumor cells that are used as the fusion partner. In the clinical trials, clinical and immunological responses were observed in patients with advanced stage of malignant tumors after being vaccinated with DC/tumor fusion cells, although the antitumor effect is not as vigorous as in the animal tumor models. This review summarizes recent advances in concepts and techniques that are providing new impulses to DCs/tumor fusions-based cancer vaccination.
Figures
References
-
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. - PubMed
-
- Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nature Reviews Immunology. 2005;5(4):296–306. - PubMed
-
- Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nature Medicine. 1998;4(3):328–332. - PubMed
-
- Koido S, Kashiwaba M, Chen D, Gendler S, Kufe D, Gong J. Induction of antitumor immunity by vaccination of dendritic cells transfected with MUC1 RNA. Journal of Immunology. 2000;165(10):5713–5719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

