Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). Possible enhancement of therapeutic potential of MSC
- PMID: 20182548
- PMCID: PMC2825653
- DOI: 10.1155/2010/795385
Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). Possible enhancement of therapeutic potential of MSC
Abstract
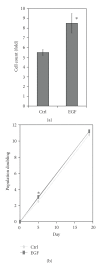
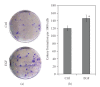
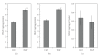
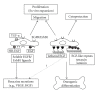
Adult bone marrow multipotential stromal cells (MSCs) hold great promise in regenerative medicine and tissue engineering. However, due to their low numbers upon harvesting, MSCs need to be expanded in vitro without biasing future differentiation for optimal utility. In this concept paper, we focus on the potential use of epidermal growth factor (EGF), prototypal growth factor for enhancing the harvesting and/or differentiation of MSCs. Soluble EGF was shown to augment MSC proliferation while preserving early progenitors within MSC population, and thus did not induce differentiation. However, tethered form of EGF was shown to promote osteogenic differentiation. Soluble EGF was also shown to increase paracrine secretions including VEGF and HGF from MSC. Thus, soluble EGF can be used not only to expand MSC in vitro, but also to enhance paracrine secretion through drug-releasing MSC-encapsulated scaffolds in vivo. Tethered EGF can also be utilized to direct MSC towards osteogenic lineage both in vitro and in vivo.
Figures

References
-
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. International Journal of Biochemistry and Cell Biology. 2004;36(4):568–584. - PubMed
-
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25(11):2896–2902. - PubMed
-
- Tamama K, Fan VH, Griffith LG, Blair HC, Wells A. Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24(3):686–695. - PubMed
-
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

