Rho and Ras GTPases in axon growth, guidance, and branching
- PMID: 20182621
- PMCID: PMC2828272
- DOI: 10.1101/cshperspect.a001818
Rho and Ras GTPases in axon growth, guidance, and branching
Abstract
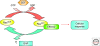
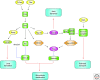
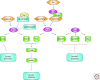
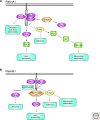
The establishment of precise neuronal cell morphology provides the foundation for all aspects of neurobiology. During development, axons emerge from cell bodies after an initial polarization stage, elongate, and navigate towards target regions guided by a range of environmental cues. The Rho and Ras families of small GTPases have emerged as critical players at all stages of axonogenesis. Their ability to coordinately direct multiple signal transduction pathways with precise spatial control drives many of the activities that underlie this morphogenetic program: the dynamic assembly, disassembly, and reorganization of the actin and microtubule cytoskeletons, the interaction of the growing axon with other cells and extracellular matrix, the delivery of lipids and proteins to the axon through the exocytic machinery, and the internalization of membrane and proteins at the leading edge of the growth cone through endocytosis. This article highlights the contribution of Rho and Ras GTPases to axonogenesis.
Figures
References
-
- Acebes A, Ferrus A 2000. Cellular and molecular features of axon collaterals and dendrites. Trends Neurosci 23:557–565 - PubMed
-
- Ahnert-Hilger G, Holtje M, Grosse G, Pickert G, Mucke C, Nixdorf-Bergweiler B, Boquet P, Hofmann F, Just I 2004. Differential effects of Rho GTPases on axonal and dendritic development in hippocampal neurones. J Neurochem 90:9–18 - PubMed
-
- Allen MJ, Shan X, Murphey RK 2000. A role for Drosophila Drac1 in neurite outgrowth and synaptogenesis in the giant fiber system. Mol Cell Neurosci 16:754–765 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
