Guiding neuronal cell migrations
- PMID: 20182622
- PMCID: PMC2828271
- DOI: 10.1101/cshperspect.a001834
Guiding neuronal cell migrations
Abstract
Neuronal migration is, along with axon guidance, one of the fundamental mechanisms underlying the wiring of the brain. As other organs, the nervous system has acquired the ability to grow both in size and complexity by using migration as a strategy to position cell types from different origins into specific coordinates, allowing for the generation of brain circuitries. Guidance of migrating neurons shares many features with axon guidance, from the use of substrates to the specific cues regulating chemotaxis. There are, however, important differences in the cell biology of these two processes. The most evident case is nucleokinesis, which is an essential component of migration that needs to be integrated within the guidance of the cell. Perhaps more surprisingly, the cellular mechanisms underlying the response of the leading process of migrating cells to guidance cues might be different to those involved in growth cone steering, at least for some neuronal populations.
Figures

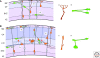
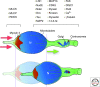
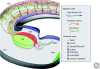

References
-
- Abercrombie M, Heaysman J 1953. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp Cell Res 5:111–131 - PubMed
-
- Adams NC, Tomoda T, Cooper M, Dietz G, Hatten ME 2002. Mice that lack astrotactin have slowed neuronal migration. Development 129:965–972 - PubMed
-
- Angevine JB, Sidman RL 1961. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192:766–768 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
