Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling
- PMID: 20182623
- PMCID: PMC2828280
- DOI: 10.1101/cshperspect.a002915
Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling
Abstract
The epithelial-mesenchymal transition is essential in both embryonic development and the progression of carcinomas. Wnt signaling and cadherin-mediated adhesion have been implicated in both processes; clarifying their role will depend on linking them to rearrangements of cellular structure and behavior. beta-Catenin is an essential molecule both in cadherin-mediated cell adhesion and in canonical Wnt signaling. Numerous experiments have shown that the loss of cadherin-mediated cell adhesion can promote beta-catenin release and signaling; this is accomplished by proteases, protein kinases and other molecules. Cadherin loss can also signal to several other regulatory pathways. Additionally, many target genes of Wnt signaling influence cadherin adhesion. The most conspicuous of these Wnt target genes encode the transcription factors Twist and Slug, which directly inhibit the E-cadherin gene promoter. Other Wnt/beta-catenin target genes encode metalloproteases or the cell adhesion molecule L1, which favor the degradation of E-cadherin. These factors provide a mechanism whereby cadherin loss and increased Wnt signaling induce epithelial-mesenchymal transition in both carcinomas and development.
Figures
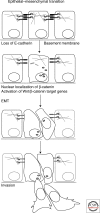



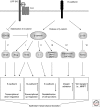
References
-
- Abe K, Takeichi M 2007. NMDA-receptor activation induces calpain-mediated β-catenin cleavages for triggering gene expression. Neuron 53:387–397 - PubMed
-
- Adachi S, Jigami T, Yasui T, Nakano T, Ohwada S, Omori Y, Sugano S, Ohkawara B, Shibuya H, Nakamura T, et al.2004. Role of a BCL9-related β-catenin-binding protein, B9L, in tumorigenesis induced by aberrant activation of Wnt signaling. Cancer Res 64:8496–8501 - PubMed
-
- Alexander NR, Tran NL, Rekapally H, Summers CE, Glackin C, Heimark RL 2006. N-cadherin gene expression in prostate carcinoma is modulated by integrin-dependent nuclear translocation of Twist1. Cancer Res 66:3365–3369 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
