Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis
- PMID: 20182627
- PMCID: PMC2825658
- DOI: 10.1155/2010/453892
Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis
Abstract
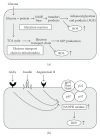
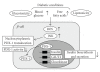
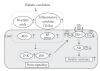
Type 2 diabetes is the most prevalent and serious metabolic disease all over the world, and its hallmarks are pancreatic beta-cell dysfunction and insulin resistance. Under diabetic conditions, chronic hyperglycemia and subsequent augmentation of reactive oxygen species (ROS) deteriorate beta-cell function and increase insulin resistance which leads to the aggravation of type 2 diabetes. In addition, chronic hyperglycemia and ROS are also involved in the development of atherosclerosis which is often observed under diabetic conditions. Taken together, it is likely that ROS play an important role in the development of type 2 diabetes and atherosclerosis.
Figures

References
-
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48(1):1–9. - PubMed
-
- Dandona P, Thusu K, Cook S, et al. Oxidative damage to DNA in diabetes mellitus. The Lancet. 1996;347(8999):444–445. - PubMed
-
- Sakurai T, Tsuchiya S. Superoxide production from nonenzymatically glycated protein. FEBS Letters. 1988;236(2):406–410. - PubMed
-
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. - PubMed
-
- Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. The American Journal of Cardiology. 2003;91(3):7A–11A. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

