Freeze thaw: a simple approach for prediction of optimal cryoprotectant for freeze drying
- PMID: 20182826
- PMCID: PMC2850490
- DOI: 10.1208/s12249-010-9382-3
Freeze thaw: a simple approach for prediction of optimal cryoprotectant for freeze drying
Abstract
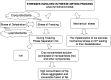
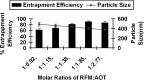
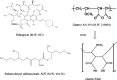
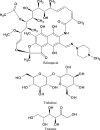
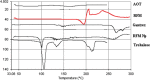
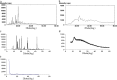
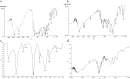
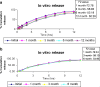
The present study evaluates freeze thaw as a simple approach for screening the most appropriate cryoprotectant. Freeze-thaw study is based on the principle that an excipient, which protects nanoparticles during the first step of freezing, is likely to be an effective cryoprotectant. Nanoparticles of rifampicin with high entrapment efficiency were prepared by the emulsion-solvent diffusion method using dioctyl sodium sulfosuccinate (AOT) as complexing agent and Gantrez AN-119 as polymer. Freeze-thaw study was carried out using trehalose and fructose as cryoprotectants. The concentration of cryoprotectant, concentration of nanoparticles in the dispersion, and the freezing temperature were varied during the freeze-thaw study. Cryoprotection increased with increase in cryoprotectant concentration. Further, trehalose was superior to fructose at equivalent concentrations and moreover permitted use of more concentrated nanosuspensions for freeze drying. Freezing temperature did not influence the freeze-thaw study. Freeze-dried nanoparticles revealed good redispersibility with a size increase that correlated well with the freeze-thaw study at 20% w/v trehalose and fructose. Transmission electron microscopy revealed round particles with a size approximately 400 nm, which correlated with photon correlation spectroscopic measurements. Differential scanning calorimetry and X-ray diffraction suggested amorphization of rifampicin. Fourier transfer infrared spectroscopy could not confirm interaction of drug with AOT. Nanoparticles exhibited sustained release of rifampicin, which followed diffusion kinetics. Nanoparticles of rifampicin were found to be stable for 12 months. The good correlation between freeze thaw and freeze drying suggests freeze-thaw study as a simple and quick approach for screening optimal cryoprotectant for freeze drying.
Figures


Similar articles
-
Cryoprotectants for freeze drying of drug nano-suspensions: effect of freezing rate.J Pharm Sci. 2009 Dec;98(12):4808-17. doi: 10.1002/jps.21786. J Pharm Sci. 2009. PMID: 19475555
-
Study on formability of solid nanosuspensions during solidification: II novel roles of freezing stress and cryoprotectant property.Int J Pharm. 2014 Nov 20;475(1-2):35-48. doi: 10.1016/j.ijpharm.2014.08.041. Epub 2014 Aug 23. Int J Pharm. 2014. PMID: 25158243
-
Freeze-drying of nanosuspensions, 1: freezing rate versus formulation design as critical factors to preserve the original particle size distribution.J Pharm Sci. 2011 May;100(5):1958-68. doi: 10.1002/jps.22425. Epub 2010 Dec 10. J Pharm Sci. 2011. PMID: 21374626
-
Applications of Freezing and Freeze-Drying in Pharmaceutical Formulations.Adv Exp Med Biol. 2018;1081:371-383. doi: 10.1007/978-981-13-1244-1_20. Adv Exp Med Biol. 2018. PMID: 30288720 Review.
-
Fundamentals of freeze-drying.Pharm Biotechnol. 2002;14:281-360. doi: 10.1007/978-1-4615-0549-5_6. Pharm Biotechnol. 2002. PMID: 12189727 Review.
Cited by
-
Protective Effect of Saccharides on Freeze-Dried Liposomes Encapsulating Drugs.Front Bioeng Biotechnol. 2019 Dec 17;7:424. doi: 10.3389/fbioe.2019.00424. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31921827 Free PMC article.
-
Rivastigmine-loaded L-lactide-depsipeptide polymeric nanoparticles: decisive formulation variable optimization.Sci Pharm. 2013 Mar 28;81(3):865-85. doi: 10.3797/scipharm.1211-20. Print 2013 Jul-Sep. Sci Pharm. 2013. PMID: 24106679 Free PMC article.
-
Development of Houttuynia cordata Extract-Loaded Solid Lipid Nanoparticles for Oral Delivery: High Drug Loading Efficiency and Controlled Release.Molecules. 2017 Dec 13;22(12):2215. doi: 10.3390/molecules22122215. Molecules. 2017. PMID: 29236057 Free PMC article.
-
Enhancing the Stabilization Potential of Lyophilization for Extracellular Vesicles.Adv Healthc Mater. 2022 Mar;11(5):e2100538. doi: 10.1002/adhm.202100538. Epub 2021 Jul 26. Adv Healthc Mater. 2022. PMID: 34310074 Free PMC article.
-
Modification of microneedles using inkjet printing.AIP Adv. 2011 Jun;1(2):22139. doi: 10.1063/1.3602461. Epub 2011 Jun 10. AIP Adv. 2011. PMID: 22125759 Free PMC article.
References
-
- Rodriguez GS, Allémann E, Fessi H, Doelker E. Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharm Res. 2004;21:1428–1439. doi: 10.1023/B:PHAM.0000036917.75634.be. - DOI - PubMed
-
- Oppenheim R. Solid colloidal drug delivery systems: nanoparticles. Int J Pharm. 1981;8:217–234. doi: 10.1016/0378-5173(81)90100-9. - DOI
-
- Alonso MJ. Nanoparticulate drug carrier technology. In: Cohen S, Bernstein H, editors. Microparticulate systems for the delivery of proteins and vaccines. New York: Marcel Dekker; 1996. pp. 203–242.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

