In vitro release kinetics and bioavailability of gastroretentive cinnarizine hydrochloride tablet
- PMID: 20182827
- PMCID: PMC2850455
- DOI: 10.1208/s12249-010-9380-5
In vitro release kinetics and bioavailability of gastroretentive cinnarizine hydrochloride tablet
Abstract
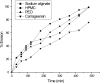
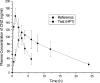
An oral sustained release dosage form of cinnarizine HCl (CNZ) based on gastric floating matrix tablets was studied. The release of CNZ from different floating matrix formulations containing four viscosity grades of hydroxypropyl methylcellulose, sodium alginate or polyethylene oxide, and gas-forming agent (sodium bicarbonate or calcium carbonate) was studied in simulated gastric fluid (pH 1.2). CNZ release data from the matrix tablets were analyzed kinetically using Higuchi, Peppas, Weibull, and Vergnaud models. From water uptake, matrix erosion studies, and drug release data, the overall release mechanism can be explained as a result of rapid hydration of polymer on the surface of the floating tablet and formation of a gel layer surrounding the matrix that controls water penetration into its center. On the basis of in vitro release data, batch HP1 (CNZ, HPMC-K100LV, SBC, LTS, and MgS) was subjected to bioavailability studies in rabbits and was compared with CNZ suspension. It was concluded that the greater bioavailability of HP1 was due to its longer retention in the gastric environment of the test animal. Batch no. HP1 of floating tablet in rabbits demonstrated that the floating tablet CNZ could be a 24-h sustained release formulation.
Figures





Similar articles
-
Enhanced bioavailability of atorvastatin calcium from stabilized gastric resident formulation.AAPS PharmSciTech. 2011 Dec;12(4):1077-86. doi: 10.1208/s12249-011-9673-3. Epub 2011 Aug 31. AAPS PharmSciTech. 2011. PMID: 21879394 Free PMC article.
-
Gastroretentive Matrix Tablets of Boswellia Oleogum Resin: Preparation, Optimization, In Vitro Evaluation, and Cytoprotective Effect on Indomethacin-Induced Gastric Ulcer in Rabbits.AAPS PharmSciTech. 2016 Apr;17(2):328-38. doi: 10.1208/s12249-015-0351-8. Epub 2015 Jun 20. AAPS PharmSciTech. 2016. PMID: 26092303 Free PMC article.
-
Preparation and evaluation of gastroretentive floating tablets of Silymarin.Chem Pharm Bull (Tokyo). 2009 Jun;57(6):545-9. doi: 10.1248/cpb.57.545. Chem Pharm Bull (Tokyo). 2009. PMID: 19483331
-
Alginate-based matrix tablets for drug delivery.Expert Opin Drug Deliv. 2023 Jan;20(1):115-130. doi: 10.1080/17425247.2023.2158183. Epub 2022 Dec 18. Expert Opin Drug Deliv. 2023. PMID: 36503355 Review.
-
HPMC- A Marvel Polymer for Pharmaceutical Industry-Patent Review.Recent Adv Drug Deliv Formul. 2021;15(1):46-58. doi: 10.2174/1872211314666210604120619. Recent Adv Drug Deliv Formul. 2021. PMID: 34086557 Review.
Cited by
-
Novel Mucoadhesive Chitosomes as a Platform for Enhanced Oral Bioavailability of Cinnarizine.Int J Nanomedicine. 2022 Nov 24;17:5641-5660. doi: 10.2147/IJN.S384494. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36452306 Free PMC article.
-
Natural gums as sustained release carriers: development of gastroretentive drug delivery system of ziprasidone HCl.Daru. 2012 Oct 17;20(1):58. doi: 10.1186/2008-2231-20-58. Daru. 2012. PMID: 23352292 Free PMC article.
-
Development and characterization of gastroretentive sustained-release formulation by combination of swelling and mucoadhesive approach: a mechanistic study.Drug Des Devel Ther. 2013 Dec 5;7:1455-69. doi: 10.2147/DDDT.S52890. eCollection 2013. Drug Des Devel Ther. 2013. PMID: 24348022 Free PMC article.
-
Preparation and characterization of a gastric floating dosage form of capecitabine.Biomed Res Int. 2013;2013:495319. doi: 10.1155/2013/495319. Epub 2013 Oct 29. Biomed Res Int. 2013. PMID: 24288681 Free PMC article.
-
Recent advances in delivery systems and therapeutics of cinnarizine: a poorly water soluble drug with absorption window in stomach.J Drug Deliv. 2014;2014:479246. doi: 10.1155/2014/479246. Epub 2014 Nov 13. J Drug Deliv. 2014. PMID: 25478230 Free PMC article. Review.
References
-
- Hwang SJ, Park H, Park K. Gastric retentive drug-delivery systems. Crit Rev Ther Drug Carrier Syst. 1998;15:243–284. - PubMed
-
- Hilton AK, Deasy PB. In vitro and in vivo evaluation of an oral sustained-release floating dosage form of amoxycillin trihydrate. Int J Pharm. 1992;86:79–88. doi: 10.1016/0378-5173(92)90033-X. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

