Comparison of the prophylactic effect of silymarin and deferoxamine on iron overload-induced hepatotoxicity in rat
- PMID: 20182837
- PMCID: PMC3550439
- DOI: 10.1007/s13181-010-0030-9
Comparison of the prophylactic effect of silymarin and deferoxamine on iron overload-induced hepatotoxicity in rat
Abstract
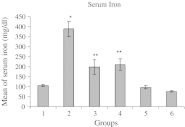
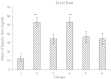
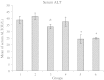
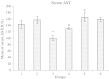
In pathologic conditions or poisoning states, iron overload can affect different tissues including liver. In this study, the prophylactic effect of deferoxamine and silymarin was compared in decreasing experimental iron-overload-induced hepatotoxicity in rats. The study was done in six groups of rats, which received drugs q2 days for 2 weeks. The rats in groups 1 to 6 received drugs, respectively: normal saline, iron dextran, iron dextran + deferoxamine (intraperitoneally), iron dextran + silymarin (orally), iron dextran + silymarin (intraperitoneally), and iron dextran + deferoxamine (intraperitoneally) + silymarin (intraperitoneally). At the end of the study, blood was collected, and serum was separated for laboratory tests. The liver of rats was separated for iron measuring and tissue processing. The serum iron concentration and the serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity were determined. The numbers of necrotic hepatocytes were counted as quantity index tissue injury in light microscopic examination. The mean of serum and liver iron in group 2 was significantly greater than group 1. Liver iron was significantly decreased in other groups except group 4. Also serum iron was decreased in groups 3 to 6 compared to group 2 (nearly 400%). ALT activity in group 3 and AST activity in group 5 were significantly lesser than in other groups. The mean of necrotic hepatocytes in group 2 was significantly increased in comparison to group 1. This elevation was significantly prevented by deferoxamine and silymarin. The result of the present study shows that silymarin has a protective effect similar to deferoxamine on iron overload-induced hepatotoxicity.
Figures

References
-
- Abu Ghadeer AR, Ali SE, Osman SA, Abu Bedair FA, Abbady MM, El-Kady MR. Antagonistic role of silymarin against cardiotoxicity and impaired antioxidant induced by adriamycin and/or radiation exposure in albino rats. Pakistan J Biol Sci. 2001;4:604–607. doi: 10.3923/pjbs.2001.604.607. - DOI
-
- Ahmed B, Khan SA, Alam T. Synthesis and antihepatotoxic activity of some heterocyclic compounds containing the 1, 4-dioxane ring system. Pharmazie. 2003;58:173–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

