Growth inhibition of tumor cells in vitro by using monoclonal antibodies against gonadotropin-releasing hormone receptor
- PMID: 20182875
- PMCID: PMC11030974
- DOI: 10.1007/s00262-010-0823-3
Growth inhibition of tumor cells in vitro by using monoclonal antibodies against gonadotropin-releasing hormone receptor
Abstract
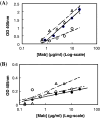
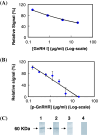
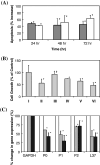
As the continuation of a previous study, synthetic peptides corresponding to the extracellular domains of human gonadotropin-releasing hormone (GnRH) receptor were used to generate additional monoclonal antibodies which were further characterized biochemically and immunologically. Among those identified to recognize GnRH receptor, monoclonal antibodies designated as GHR-103, GHR-106 and GHR-114 were found to exhibit high affinity (Kd < or = 1 x 10(-8) M) and specificity to GnRH receptor as judged by the whole cell binding immunoassay and Western blot assay. Both anti-GnRH receptor monoclonal antibodies and GnRH were shown to compete for the same binding site of GnRH receptor on the surface of cultured cancer cells. Growth inhibitions of cancer cells cultured in vitro were demonstrated by cellular apoptosis experiments (TUNEL and MTT assays) under different conditions of treatment with GHR-106 monoclonal antibody or GnRH analogs. It was generally observed that both GnRH I and GHR-106 effectively induce the apoptosis of cultured cancer cells as determined by TUNEL and MTT assays. Consistently, suppressions of gene expressions at mRNA levels were demonstrated with several ribosomal proteins (P0, P1, P2 and L37), when cancer cells were incubated with GnRH or GHR-106. The widespread expressions of GnRH receptor in almost all of the studied human cancer cell lines were also demonstrated by RT-PCR and Western blot assay, as well as indirect immunofluorescence assay with either of these monoclonal antibodies as the primary antibody. In view of the longer half life of antibodies as compared to that of GnRH or its analogs, anti-GnRH receptor monoclonal antibodies in humanized forms could function as GnRH analogs and serve as an ideal candidate of anti-cancer drugs for therapeutic treatments of various cancers in humans as well as for fertility regulations.
Figures

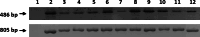
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

