Base-functionalized carbocyclic nucleosides: design, synthesis, and mechanism of antiviral activity
- PMID: 20183592
- PMCID: PMC2829736
- DOI: 10.1080/15257770903044465
Base-functionalized carbocyclic nucleosides: design, synthesis, and mechanism of antiviral activity
Abstract
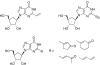
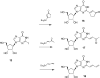
New carbocyclic ribonucleosides with unsaturated groups at the C-2 position of the nucleobase were designed as potential RNA antiviral compounds. The design was based on the expectation that the monophosphates of these compounds would be inhibitors of the enzyme, IMPDH. Appropriate methodologies were developed to achieve the target molecules. Results from the initial in vitro antiviral studies are mentioned. The IMPDH-associated mechanism of the antiviral activity of the most active compound is supported by enzyme inhibition studies.
Figures





Similar articles
-
IMPDH as a biological probe for RNA antiviral drug discovery: synthesis, enzymology, molecular docking, and antiviral activity of new ribonucleosides with surrogate bases.Nucleosides Nucleotides Nucleic Acids. 2007;26(6-7):651-4. doi: 10.1080/15257770701490506. Nucleosides Nucleotides Nucleic Acids. 2007. PMID: 18066873
-
Ribonucleosides bearing Michael acceptors: synthesis, molecular docking, enzyme inhibition data and antiviral activity.Nucleic Acids Symp Ser (Oxf). 2008;(52):625-6. doi: 10.1093/nass/nrn316. Nucleic Acids Symp Ser (Oxf). 2008. PMID: 18776535 No abstract available.
-
2'-Deoxy-2'-C-trifluoromethyl beta-D-ribonucleoside analogues: synthesis and antiviral evaluations.Nucleosides Nucleotides Nucleic Acids. 2003 May-Aug;22(5-8):857-9. doi: 10.1081/NCN-120022671. Nucleosides Nucleotides Nucleic Acids. 2003. PMID: 14565296
-
Nucleoside and non-nucleoside IMP dehydrogenase inhibitors as antitumor and antiviral agents.Curr Med Chem. 1999 Jul;6(7):599-614. Curr Med Chem. 1999. PMID: 10390603 Review.
-
Inosine monophosphate dehydrogenase as a probe in antiviral drug discovery.Antivir Chem Chemother. 2007;18(5):245-58. doi: 10.1177/095632020701800501. Antivir Chem Chemother. 2007. PMID: 18046958 Review.
Cited by
-
Synthesis of a novel carbocyclic analog of bredinin.Molecules. 2013 Sep 17;18(9):11576-85. doi: 10.3390/molecules180911576. Molecules. 2013. PMID: 24048288 Free PMC article.
-
Modified Nucleotides for Discrimination between Cytosine and the Epigenetic Marker 5-Methylcytosine.Angew Chem Int Ed Engl. 2016 Feb 24;55(9):3229-32. doi: 10.1002/anie.201511520. Epub 2016 Feb 2. Angew Chem Int Ed Engl. 2016. PMID: 26835661 Free PMC article.
References
-
- Weber G, Nakamura H, Natsumeda Y, Szekeres T, Nagai M. Regulation of GTP biosynthesis. Adv. Enzyme Regu. 1992;32:57–69. - PubMed
-
- Natsumeda Y, Ohno S, Kawasaki H, Konno Y, Weber G, Suzuki K. Two distinct cDNAs for human IMP dehydrogenase. J. Biol. Chem. 1990;265:5292–5295. - PubMed
-
- Collart FR, Huberman E. Cloning and sequence analysis of the human and Chinese hamster inosine-5′-monophosphate dehydrogenase cDNAs. J. Biol. Chem. 1988;263:15769–15772. - PubMed
-
- Senda M, Natsumeda Y. Tissue-differential expression of two distinct genes for human IMP dehydrogenase (E.C.1.1.1.205) Life Sciences. 1994;54:1917–1926. - PubMed
-
- Nagai M, Natsumeda Y, Konno Y, Hoffman R, Irino S, Weber G. Selective up-regulation of type II inosine 5'-monophosphate dehydrogenase messenger RNA expression in human leukemias. Cancer Res. 1991;51:3886–3890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
