Inhibitory properties of the P1 Tyr variant of antithrombin
- PMID: 20184328
- PMCID: PMC2844450
- DOI: 10.1021/bi100120a
Inhibitory properties of the P1 Tyr variant of antithrombin
Abstract
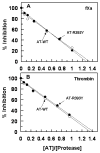
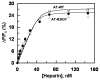
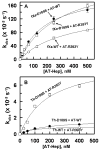
Antithrombin (AT) and protein Z-dependent protease inhibitor (ZPI) are among two physiological serpin inhibitors in plasma that are involved in the regulation of the clotting cascade. Unlike AT, which can inhibit the proteolytic activity of all coagulation proteases, ZPI has narrower protease specificity, inhibiting only factors Xa (fXa) and XIa. Unlike an Arg at the P1 site of the AT reactive center loop (RCL), this residue is a Tyr in ZPI. To investigate the contribution of P1 Tyr in restricting the specificity of ZPI, we engineered an AT mutant in which the P1 Arg of the RCL was replaced with the P1 Tyr of ZPI (AT-R393Y). The reactivity of AT-R393Y with fXa and thrombin was decreased 155- and 970-fold, respectively. However, the serpin mutant inhibited chymotrypsin with an efficiency higher by >4 orders of magnitude. By contrast, chymotrypsin did not exhibit any reactivity with ZPI. The substitution of Asp-189 of fXa with the corresponding residue of chymotrypsin (Ser) did not improve the reactivity of the protease mutant with AT-R393Y; however, the fXa mutant reacted normally with ZPI. These results suggest that the contribution of P1 Tyr to restricting the protease specificity of ZPI is RCL context-dependent and that in addition to P1 Tyr, other structural features within and/or outside the ZPI RCL are involved in determining the protease specificity of the serpin. The results further suggest that thrombin is less tolerant than fXa in accommodating the nonoptimal P1 Tyr of the AT mutant in its active-site pocket.
Figures
Similar articles
-
The antithrombin P1 residue is important for target proteinase specificity but not for heparin activation of the serpin. Characterization of P1 antithrombin variants with altered proteinase specificity but normal heparin activation.Biochemistry. 2001 Jun 5;40(22):6670-9. doi: 10.1021/bi002933d. Biochemistry. 2001. PMID: 11380262
-
Characterization of Protein Z-Dependent Protease Inhibitor/Antithrombin Chimeras Provides Insight into the Serpin Specificity of Coagulation Proteases.ACS Omega. 2017 Jul 31;2(7):3276-3283. doi: 10.1021/acsomega.7b00606. Epub 2017 Jul 7. ACS Omega. 2017. PMID: 28782047 Free PMC article.
-
Basis for the specificity and activation of the serpin protein Z-dependent proteinase inhibitor (ZPI) as an inhibitor of membrane-associated factor Xa.J Biol Chem. 2010 Jun 25;285(26):20399-409. doi: 10.1074/jbc.M110.112748. Epub 2010 Apr 28. J Biol Chem. 2010. PMID: 20427285 Free PMC article.
-
Determinants of specificity of factor xa interaction with its physiological inhibitors.Mini Rev Med Chem. 2006 Aug;6(8):859-65. doi: 10.2174/138955706777935017. Mini Rev Med Chem. 2006. PMID: 16918492 Review.
-
Protein Z-dependent regulation of coagulation.Thromb Haemost. 2001 Jul;86(1):8-13. Thromb Haemost. 2001. PMID: 11487045 Review.
References
-
- Schechter I, Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967;27:157–162. - PubMed
-
- Damus PS, Hicks M, Rosenberg RD. Anticoagulant action of heparin. Nature. 1973;246:355–357. - PubMed
-
- Gettins PGW. Serpins structure, mechanism, and function. Chem Rev. 2002;102:4751–4803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

