Mass spectroscopy and molecular modeling predict endothelial nitric oxide synthase dimer collapse by hydrogen peroxide through zinc tetrathiolate metal-binding site disruption
- PMID: 20184449
- PMCID: PMC2883531
- DOI: 10.1089/dna.2009.0858
Mass spectroscopy and molecular modeling predict endothelial nitric oxide synthase dimer collapse by hydrogen peroxide through zinc tetrathiolate metal-binding site disruption
Abstract
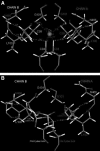
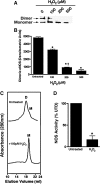
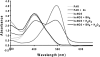
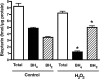
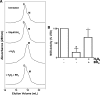
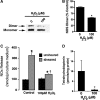
Endothelial nitric oxide synthase (eNOS) is inhibited by hydrogen peroxide (H(2)O(2)), but the mechanism has not been determined. Thus, the purpose of this study was to delineate the mechanism by which H(2)O(2) inhibits eNOS activity. Using mass spectroscopy, we found that the tetrathiolate cysteine residues 94 and 99 were susceptible to oxidation by H(2)O(2). Molecular modeling predicted that these cysteic acid modifications would disrupt the van der Waals interactions and the hydrogen bonding network mediated by the tetrathiolate cysteines 94 and 99 resulting in changes in quaternary structure, zinc release, and dimer collapse. Using recombinant human eNOS (heNOS) to test the predictions of the molecular modeling we found that H(2)O(2) caused disruption of the heNOS dimer and this was accompanied by zinc release and decreased NO generation. We also found that H(2)O(2) increased the oxidation of tetrahydrobiopterin (BH(4)) to dihydrobiopterin (BH(2)), whereas preincubation of heNOS with excess BH(4) prevented the destruction of zinc tetrathiolate and dimer collapse and preserved activity. Interestingly, we found that the dimmer-stabilizing effect of BH(4) is due to its ability to act as a catalase mimetic. Further, we confirmed that, in ovine aortic endothelial cells, H(2)O(2) could also induce dimer collapse and that increasing cellular BH(4) levels could maintain eNOS in its dimeric form and NO signaling when cells were challenged with H(2)O(2). This study links the inhibitory action of H(2)O(2) on heNOS through the destruction of zinc tetrathiolate metal-binding site and dimer collapse both in vitro and in vivo.
Figures

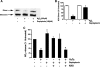
Similar articles
-
Peroxynitrite induces destruction of the tetrahydrobiopterin and heme in endothelial nitric oxide synthase: transition from reversible to irreversible enzyme inhibition.Biochemistry. 2010 Apr 13;49(14):3129-37. doi: 10.1021/bi9016632. Biochemistry. 2010. PMID: 20184376 Free PMC article.
-
Identification of the cysteine nitrosylation sites in human endothelial nitric oxide synthase.DNA Cell Biol. 2008 Jan;27(1):25-33. doi: 10.1089/dna.2007.0655. DNA Cell Biol. 2008. PMID: 17941803 Free PMC article.
-
Modeling of biopterin-dependent pathways of eNOS for nitric oxide and superoxide production.Free Radic Biol Med. 2011 Oct 1;51(7):1411-27. doi: 10.1016/j.freeradbiomed.2011.06.009. Epub 2011 Jul 8. Free Radic Biol Med. 2011. PMID: 21742028 Free PMC article.
-
eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity.J Endocrinol. 2011 Sep;210(3):271-84. doi: 10.1530/JOE-11-0083. Epub 2011 Jun 3. J Endocrinol. 2011. PMID: 21642378 Free PMC article. Review.
-
Endothelial dysfunction, endothelial nitric oxide bioavailability, tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular disease. Where are we with therapy?Microvasc Res. 2018 Sep;119:7-12. doi: 10.1016/j.mvr.2018.03.012. Epub 2018 Mar 27. Microvasc Res. 2018. PMID: 29596860 Review.
Cited by
-
Protein engineering to develop a redox insensitive endothelial nitric oxide synthase.Redox Biol. 2014;2:156-64. doi: 10.1016/j.redox.2013.12.015. Epub 2014 Jan 14. Redox Biol. 2014. PMID: 25460726 Free PMC article.
-
Mechanisms of lipotoxicity in the cardiovascular system.Curr Hypertens Rep. 2012 Dec;14(6):517-31. doi: 10.1007/s11906-012-0307-2. Curr Hypertens Rep. 2012. PMID: 23054891 Free PMC article. Review.
-
Metabolic Changes Precede the Development of Pulmonary Hypertension in the Monocrotaline Exposed Rat Lung.PLoS One. 2016 Mar 3;11(3):e0150480. doi: 10.1371/journal.pone.0150480. eCollection 2016. PLoS One. 2016. PMID: 26937637 Free PMC article.
-
Uncoupling of endothelial nitric oxide synthase in cerebral vasculature of Tg2576 mice.J Neurochem. 2015 Sep;134(6):1129-38. doi: 10.1111/jnc.13205. Epub 2015 Jul 15. J Neurochem. 2015. PMID: 26111938 Free PMC article.
-
The mitochondrial redistribution of ENOS is regulated by AKT1 and dimer status.Nitric Oxide. 2024 Nov 1;152:90-100. doi: 10.1016/j.niox.2024.09.009. Epub 2024 Sep 25. Nitric Oxide. 2024. PMID: 39332480
References
-
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. - PubMed
-
- Black S.M. Heidersbach R.S. McMullan D.M. Bekker J.M. Johengen M.J. Fineman J.R. Inhaled nitric oxide inhibits NOS activity in lambs: potential mechanism for rebound pulmonary hypertension. Am J Physiol. 1999;277:H1849–H1856. - PubMed
-
- Brennan L.A. Steinhorn R.H. Wedgwood S. Mata-Greenwood E. Roark E.A. Russell J.A. Black S.M. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res. 2003;92:683–691. - PubMed
-
- Cai S. Alp N.J. McDonald D. Smith I. Kay J. Canevari L. Heales S. Channon K.M. GTP cyclohydrolase I gene transfer augments intracellular tetrahydrobiopterin in human endothelial cells: effects on nitric oxide synthase activity, protein levels and dimerisation. Cardiovasc Res. 2002;55:838–849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

