Gliomagenesis and the use of neural stem cells in brain tumor treatment
- PMID: 20184546
- PMCID: PMC2981502
- DOI: 10.2174/187152010790909290
Gliomagenesis and the use of neural stem cells in brain tumor treatment
Abstract
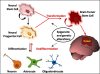
The role of neural stem cells (NSCs) in both the physiological and pathological processes in the brain has been refined through recent studies within the neuro-oncological field. Alterations in NSC regulatory mechanisms may be fundamental for the development and progression of malignant gliomas. A subpopulation of cells within the tumor known as brain tumor stem cells (BTSCs) have been shown to share key properties with NSCs. The BTSC hypothesis has significantly contributed to a potential understanding as to why brain tumors hold such dismal prognosis. On the other hand, the normal NSCs possess the capacity to migrate extensively towards the tumor bulk as well as to lingering neoplastic regions of the brain. The tropism of NSCs towards brain tumors may provide an additional tool for the treatment of brain cancer. The creation of potential therapies through the use of NSCs has been studied and includes the delivery of gene products to specific locations of the central nervous system selectively targeting malignant brain tumor cells and maximizing the efficiency of their delivery. Here, the proposed mechanisms of how brain tumors emerge, the molecular pathways interrupted in NSC pathogenesis and the most recent preclinical results in the use of NSCs for glioma treatment are reviewed.
Figures


References
-
- CBTRUS . Primary Brain Tumors in the United States. Central Brain Tumor Registry of the United States; 2007–2008.
-
- Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15(11):1311–1333. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

