Rearing environment affects development of the immune system in neonates
- PMID: 20184618
- PMCID: PMC2883114
- DOI: 10.1111/j.1365-2249.2010.04090.x
Rearing environment affects development of the immune system in neonates
Abstract
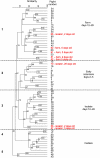
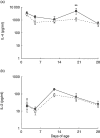
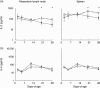
Early-life exposure to appropriate microbial flora drives expansion and development of an efficient immune system. Aberrant development results in increased likelihood of allergic disease or increased susceptibility to infection. Thus, factors affecting microbial colonization may also affect the direction of immune responses in later life. There is a need for a manipulable animal model of environmental influences on the development of microbiota and the immune system during early life. We assessed the effects of rearing under low- (farm, sow) and high-hygiene (isolator, milk formula) conditions on intestinal microbiota and immune development in neonatal piglets, because they can be removed from the mother in the first 24 h for rearing under controlled conditions and, due to placental structure, neither antibody nor antigen is transferred in utero. Microbiota in both groups was similar between 2 and 5 days. However, by 12-28 days, piglets reared on the mother had more diverse flora than siblings reared in isolators. Dendritic cells accumulated in the intestinal mucosa in both groups, but more rapidly in isolator piglets. Importantly, the minority of 2-5-day-old farm piglets whose microbiota resembled that of an older (12-28-day-old) pig also accumulated dendritic cells earlier than the other farm-reared piglets. Consistent with dendritic cell control of T cell function, the effects on T cells occurred at later time-points, and mucosal T cells from high-hygiene, isolator pigs made less interleukin (IL)-4 while systemic T cells made more IL-2. Neonatal piglets may be a valuable model for studies of the effects of interaction between microbiota and immune development on allergy.
Figures
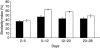

Similar articles
-
Direct experimental evidence that early-life farm environment influences regulation of immune responses.Pediatr Allergy Immunol. 2012 May;23(3):265-9. doi: 10.1111/j.1399-3038.2011.01258.x. Epub 2012 Feb 2. Pediatr Allergy Immunol. 2012. PMID: 22300455
-
Restricting microbial exposure in early life negates the immune benefits associated with gut colonization in environments of high microbial diversity.PLoS One. 2011;6(12):e28279. doi: 10.1371/journal.pone.0028279. Epub 2011 Dec 22. PLoS One. 2011. PMID: 22216092 Free PMC article.
-
Development of Immune Cells in the Intestinal Mucosa Can Be Affected by Intensive and Extensive Farm Environments, and Antibiotic Use.Front Immunol. 2018 May 16;9:1061. doi: 10.3389/fimmu.2018.01061. eCollection 2018. Front Immunol. 2018. PMID: 29868021 Free PMC article.
-
The development of the mucosal immune system pre- and post-weaning: balancing regulatory and effector function.Proc Nutr Soc. 2005 Nov;64(4):451-7. doi: 10.1079/pns2005452. Proc Nutr Soc. 2005. PMID: 16313686 Review.
-
Regulation of mucosal immune responses in effector sites.Proc Nutr Soc. 2001 Nov;60(4):427-35. doi: 10.1079/pns2001118. Proc Nutr Soc. 2001. PMID: 12069394 Review.
Cited by
-
Interactions between host and gut microbiota in domestic pigs: a review.Gut Microbes. 2020 May 3;11(3):310-334. doi: 10.1080/19490976.2019.1690363. Epub 2019 Nov 24. Gut Microbes. 2020. PMID: 31760878 Free PMC article. Review.
-
Intrauterine Growth Restriction Impairs Small Intestinal Mucosal Immunity in Neonatal Piglets.J Histochem Cytochem. 2014 Jul;62(7):510-8. doi: 10.1369/0022155414532655. Epub 2014 Apr 7. J Histochem Cytochem. 2014. PMID: 24710659 Free PMC article.
-
Comparison and Correlation Analysis of Immune Function and Gut Microbiota of Broiler Chickens Raised in Double-Layer Cages and Litter Floor Pens.Microbiol Spectr. 2022 Aug 31;10(4):e0004522. doi: 10.1128/spectrum.00045-22. Epub 2022 Jun 29. Microbiol Spectr. 2022. PMID: 35766494 Free PMC article.
-
Early Life Inoculation With Adult-Derived Microbiota Accelerates Maturation of Intestinal Microbiota and Enhances NK Cell Activation in Broiler Chickens.Front Vet Sci. 2020 Nov 19;7:584561. doi: 10.3389/fvets.2020.584561. eCollection 2020. Front Vet Sci. 2020. PMID: 33330708 Free PMC article.
-
Network analysis of temporal functionalities of the gut induced by perturbations in new-born piglets.BMC Genomics. 2015 Jul 29;16(1):556. doi: 10.1186/s12864-015-1733-8. BMC Genomics. 2015. PMID: 26220188 Free PMC article.
References
-
- Bjorksten B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108:516–20. - PubMed
-
- Rautava S, Kalliomaki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol. 2002;109:119–21. - PubMed
-
- Smits HH, Engering A, van der Kleij D, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–7. - PubMed
-
- Sudo N, Yu XN, Aiba Y, et al. An oral introduction of intestinal bacteria prevents the development of a long-term Th2-skewed immunological memory induced by neonatal antibiotic treatment in mice. Clin Exp Allergy. 2002;32:1112–16. - PubMed
-
- van Odijk J, Kull I, Borres MP, et al. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966–2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy. 2003;58:833–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

