The chick somitogenesis oscillator is arrested before all paraxial mesoderm is segmented into somites
- PMID: 20184730
- PMCID: PMC2836991
- DOI: 10.1186/1471-213X-10-24
The chick somitogenesis oscillator is arrested before all paraxial mesoderm is segmented into somites
Abstract
Background: Somitogenesis is the earliest sign of segmentation in the developing vertebrate embryo. This process starts very early, soon after gastrulation has initiated and proceeds in an anterior-to-posterior direction during body axis elongation. It is widely accepted that somitogenesis is controlled by a molecular oscillator with the same periodicity as somite formation. This periodic mechanism is repeated a specific number of times until the embryo acquires a defined specie-specific final number of somites at the end of the process of axis elongation. This final number of somites varies widely between vertebrate species. How termination of the process of somitogenesis is determined is still unknown.
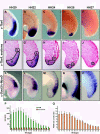
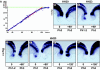
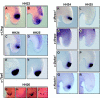
Results: Here we show that during development there is an imbalance between the speed of somite formation and growth of the presomitic mesoderm (PSM)/tail bud. This decrease in the PSM size of the chick embryo is not due to an acceleration of the speed of somite formation because it remains constant until the last stages of somitogenesis, when it slows down. When the chick embryo reaches its final number of somites at stage HH 24-25 there is still some remaining unsegmented PSM in which expression of components of the somitogenesis oscillator is no longer dynamic. Finally, we identify a change in expression of retinoic acid regulating factors in the tail bud at late stages of somitogenesis, such that in the chick embryo there is a pronounced onset of Raldh2 expression while in the mouse embryo the expression of the RA inhibitor Cyp26A1 is downregulated.
Conclusions: Our results show that the chick somitogenesis oscillator is arrested before all paraxial mesoderm is segmented into somites. In addition, endogenous retinoic acid is probably also involved in the termination of the process of segmentation, and in tail growth in general.
Figures


Similar articles
-
Somitogenesis.Curr Top Dev Biol. 1998;38:225-87. Curr Top Dev Biol. 1998. PMID: 9399080 Review.
-
Notch is a critical component of the mouse somitogenesis oscillator and is essential for the formation of the somites.PLoS Genet. 2009 Sep;5(9):e1000662. doi: 10.1371/journal.pgen.1000662. Epub 2009 Sep 25. PLoS Genet. 2009. PMID: 19779553 Free PMC article.
-
The control of somitogenesis in mouse embryos.J Embryol Exp Morphol. 1981 Oct;65 Suppl:103-28. J Embryol Exp Morphol. 1981. PMID: 6801176
-
Onset of the segmentation clock in the chick embryo: evidence for oscillations in the somite precursors in the primitive streak.Development. 2002 Mar;129(5):1107-17. doi: 10.1242/dev.129.5.1107. Development. 2002. PMID: 11874907
-
Coupling segmentation to axis formation.Development. 2004 Dec;131(23):5783-93. doi: 10.1242/dev.01519. Development. 2004. PMID: 15539483 Review.
Cited by
-
Intercellular exchange of Wnt ligands reduces cell population heterogeneity during embryogenesis.Nat Commun. 2023 Apr 6;14(1):1924. doi: 10.1038/s41467-023-37350-x. Nat Commun. 2023. PMID: 37024462 Free PMC article.
-
A spatio-temporal model of Notch signalling in the zebrafish segmentation clock: conditions for synchronised oscillatory dynamics.PLoS One. 2011 Feb 28;6(2):e16980. doi: 10.1371/journal.pone.0016980. PLoS One. 2011. PMID: 21386903 Free PMC article.
-
A Gene Regulatory Network Balances Neural and Mesoderm Specification during Vertebrate Trunk Development.Dev Cell. 2017 May 8;41(3):243-261.e7. doi: 10.1016/j.devcel.2017.04.002. Epub 2017 Apr 27. Dev Cell. 2017. PMID: 28457792 Free PMC article.
-
Uncoupling of retinoic acid signaling from tailbud development before termination of body axis extension.Genesis. 2011 Oct;49(10):776-83. doi: 10.1002/dvg.20763. Epub 2011 Aug 24. Genesis. 2011. PMID: 21538808 Free PMC article.
-
A human pluripotent stem cell-based somitogenesis model using microfluidics.Cell Stem Cell. 2024 Aug 1;31(8):1113-1126.e6. doi: 10.1016/j.stem.2024.06.004. Epub 2024 Jul 8. Cell Stem Cell. 2024. PMID: 38981471 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

