Biosynthesis of 2-hydroxyisobutyric acid (2-HIBA) from renewable carbon
- PMID: 20184738
- PMCID: PMC2847961
- DOI: 10.1186/1475-2859-9-13
Biosynthesis of 2-hydroxyisobutyric acid (2-HIBA) from renewable carbon
Abstract
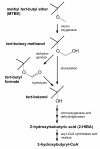
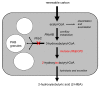
Nowadays a growing demand for green chemicals and cleantech solutions is motivating the industry to strive for biobased building blocks. We have identified the tertiary carbon atom-containing 2-hydroxyisobutyric acid (2-HIBA) as an interesting building block for polymer synthesis. Starting from this carboxylic acid, practically all compounds possessing the isobutane structure are accessible by simple chemical conversions, e. g. the commodity methacrylic acid as well as isobutylene glycol and oxide. During recent years, biotechnological routes to 2-HIBA acid have been proposed and significant progress in elucidating the underlying biochemistry has been made. Besides biohydrolysis and biooxidation, now a bioisomerization reaction can be employed, converting the common metabolite 3-hydroxybutyric acid to 2-HIBA by a novel cobalamin-dependent CoA-carbonyl mutase. The latter reaction has recently been discovered in the course of elucidating the degradation pathway of the groundwater pollutant methyl tert-butyl ether (MTBE) in the new bacterial species Aquincola tertiaricarbonis. This discovery opens the ground for developing a completely biotechnological process for producing 2-HIBA. The mutase enzyme has to be active in a suitable biological system producing 3-hydroxybutyryl-CoA, which is the precursor of the well-known bacterial bioplastic polyhydroxybutyrate (PHB). This connection to the PHB metabolism is a great advantage as its underlying biochemistry and physiology is well understood and can easily be adopted towards producing 2-HIBA. This review highlights the potential of these discoveries for a large-scale 2-HIBA biosynthesis from renewable carbon, replacing conventional chemistry as synthesis route and petrochemicals as carbon source.
Figures
Similar articles
-
Exploiting mixtures of H2, CO2, and O2 for improved production of methacrylate precursor 2-hydroxyisobutyric acid by engineered Cupriavidus necator strains.Appl Microbiol Biotechnol. 2015 Mar;99(5):2131-45. doi: 10.1007/s00253-014-6266-6. Epub 2014 Dec 12. Appl Microbiol Biotechnol. 2015. PMID: 25503317
-
Production of 2-Hydroxyisobutyric Acid from Methanol by Methylobacterium extorquens AM1 Expressing (R)-3-Hydroxybutyryl Coenzyme A-Isomerizing Enzymes.Appl Environ Microbiol. 2017 Jan 17;83(3):e02622-16. doi: 10.1128/AEM.02622-16. Print 2017 Feb 1. Appl Environ Microbiol. 2017. PMID: 27836853 Free PMC article.
-
Degradation of fuel oxygenates and their main intermediates by Aquincola tertiaricarbonis L108.Microbiology (Reading). 2008 May;154(Pt 5):1414-1421. doi: 10.1099/mic.0.2007/014159-0. Microbiology (Reading). 2008. PMID: 18451050
-
Microbial degradation and fate in the environment of methyl tert-butyl ether and related fuel oxygenates.Appl Microbiol Biotechnol. 2001 Aug;56(3-4):339-49. doi: 10.1007/s002530100647. Appl Microbiol Biotechnol. 2001. PMID: 11549000 Review.
-
Biodegradation and fate of ethyl tert-butyl ether (ETBE) in soil and groundwater: A review.J Hazard Mater. 2020 Jun 5;391:122046. doi: 10.1016/j.jhazmat.2020.122046. Epub 2020 Jan 24. J Hazard Mater. 2020. PMID: 32145642 Review.
Cited by
-
Thermophilic Coenzyme B12-Dependent Acyl Coenzyme A (CoA) Mutase from Kyrpidia tusciae DSM 2912 Preferentially Catalyzes Isomerization of (R)-3-Hydroxybutyryl-CoA and 2-Hydroxyisobutyryl-CoA.Appl Environ Microbiol. 2015 Jul;81(14):4564-72. doi: 10.1128/AEM.00716-15. Epub 2015 Apr 24. Appl Environ Microbiol. 2015. PMID: 25911482 Free PMC article.
-
Structural basis of the stereospecificity of bacterial B12-dependent 2-hydroxyisobutyryl-CoA mutase.J Biol Chem. 2015 Apr 10;290(15):9727-37. doi: 10.1074/jbc.M115.645689. Epub 2015 Feb 26. J Biol Chem. 2015. PMID: 25720495 Free PMC article.
-
Actinobacterial Degradation of 2-Hydroxyisobutyric Acid Proceeds via Acetone and Formyl-CoA by Employing a Thiamine-Dependent Lyase Reaction.Front Microbiol. 2020 Apr 15;11:691. doi: 10.3389/fmicb.2020.00691. eCollection 2020. Front Microbiol. 2020. PMID: 32351493 Free PMC article.
-
Novel B(12)-dependent acyl-CoA mutases and their biotechnological potential.Biochemistry. 2012 Aug 7;51(31):6039-46. doi: 10.1021/bi300827v. Epub 2012 Jul 23. Biochemistry. 2012. PMID: 22803641 Free PMC article. Review.
-
Assessing the intracellular primary metabolic profile of Trichoderma reesei and Aspergillus niger grown on different carbon sources.Front Fungal Biol. 2022 Sep 27;3:998361. doi: 10.3389/ffunb.2022.998361. eCollection 2022. Front Fungal Biol. 2022. PMID: 37746225 Free PMC article.
References
-
- Werpy T, Petersen G, (Eds) Top Value Added Chemicals From Biomass. US Department of Energy; 2004. http://www1.eere.energy.gov/biomass/pdfs/35523.pdf
-
- Bauer W. Ullmann's encyclopedia of industrial chemistry. 6. Vol. 21. Weinheim: Wiley-VCH; 2002. Methacrylic acid and derivatives; pp. 585–597.
-
- Chisholm MS. Artificial glass - the versatility of poly(methyl methacrylate) from its early exploitation to the new millennium. J Chem Edu. 2000;77:841–845. doi: 10.1021/ed077p841. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

