Germ band retraction as a landmark in glucose metabolism during Aedes aegypti embryogenesis
- PMID: 20184739
- PMCID: PMC2838828
- DOI: 10.1186/1471-213X-10-25
Germ band retraction as a landmark in glucose metabolism during Aedes aegypti embryogenesis
Abstract
Background: The mosquito A. aegypti is vector of dengue and other viruses. New methods of vector control are needed and can be achieved by a better understanding of the life cycle of this insect. Embryogenesis is a part of A. aegypty life cycle that is poorly understood. In insects in general and in mosquitoes in particular energetic metabolism is well studied during oogenesis, when the oocyte exhibits fast growth, accumulating carbohydrates, lipids and proteins that will meet the regulatory and metabolic needs of the developing embryo. On the other hand, events related with energetic metabolism during A. aegypti embryogenesis are unknown.
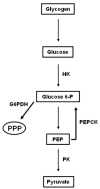
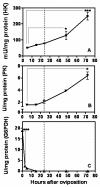
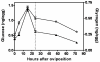
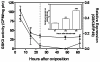
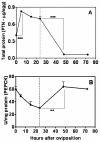
Results: Glucose metabolism was investigated throughout Aedes aegypti (Diptera) embryonic development. Both cellular blastoderm formation (CBf, 5 h after egg laying - HAE) and germ band retraction (GBr, 24 HAE) may be considered landmarks regarding glucose 6-phosphate (G6P) destination. We observed high levels of glucose 6-phosphate dehydrogenase (G6PDH) activity at the very beginning of embryogenesis, which nevertheless decreased up to 5 HAE. This activity is correlated with the need for nucleotide precursors generated by the pentose phosphate pathway (PPP), of which G6PDH is the key enzyme. We suggest the synchronism of egg metabolism with carbohydrate distribution based on the decreasing levels of phosphoenolpyruvate carboxykinase (PEPCK) activity and on the elevation observed in protein content up to 24 HAE. Concomitantly, increasing levels of hexokinase (HK) and pyruvate kinase (PK) activity were observed, and PEPCK reached a peak around 48 HAE. Glycogen synthase kinase (GSK3) activity was also monitored and shown to be inversely correlated with glycogen distribution during embryogenesis.
Conclusions: The results herein support the hypothesis that glucose metabolic fate changes according to developmental embryonic stages. Germ band retraction is a moment that was characterized as a landmark in glucose metabolism during Aedes aegypti embryogenesis. Furthermore, the results also suggest a role for GSK3 in glycogen balance/distribution during morphological modifications.
Figures


Similar articles
-
Hypometabolic strategy and glucose metabolism maintenance of Aedes aegypti egg desiccation.Comp Biochem Physiol B Biochem Mol Biol. 2019 Jan;227:56-63. doi: 10.1016/j.cbpb.2018.09.005. Epub 2018 Sep 25. Comp Biochem Physiol B Biochem Mol Biol. 2019. PMID: 30266630
-
Differential expression of PEPCK isoforms is correlated to Aedes aegypti oogenesis and embryogenesis.Comp Biochem Physiol B Biochem Mol Biol. 2021 Oct-Dec;256:110618. doi: 10.1016/j.cbpb.2021.110618. Epub 2021 May 17. Comp Biochem Physiol B Biochem Mol Biol. 2021. PMID: 34015437
-
Glucose metabolism during embryogenesis of the hard tick Boophilus microplus.Comp Biochem Physiol A Mol Integr Physiol. 2007 Apr;146(4):528-33. doi: 10.1016/j.cbpa.2006.05.009. Epub 2006 May 23. Comp Biochem Physiol A Mol Integr Physiol. 2007. PMID: 16904922
-
Metabolic phenotype of bladder cancer.Cancer Treat Rev. 2016 Apr;45:46-57. doi: 10.1016/j.ctrv.2016.03.005. Epub 2016 Mar 8. Cancer Treat Rev. 2016. PMID: 26975021 Review.
-
Proliferating tumor cells mimick glucose metabolism of mature human erythrocytes.Cell Cycle. 2019 Jun;18(12):1316-1334. doi: 10.1080/15384101.2019.1618125. Epub 2019 Jun 3. Cell Cycle. 2019. PMID: 31154896 Free PMC article. Review.
Cited by
-
Glycogen and glucose metabolism are essential for early embryonic development of the red flour beetle Tribolium castaneum.PLoS One. 2013 Jun 4;8(6):e65125. doi: 10.1371/journal.pone.0065125. Print 2013. PLoS One. 2013. PMID: 23750237 Free PMC article.
-
A global phosphoproteomics analysis of adult Fasciola gigantica by LC-MS/MS.Parasitol Res. 2022 Feb;121(2):623-631. doi: 10.1007/s00436-021-07422-2. Epub 2022 Jan 5. Parasitol Res. 2022. PMID: 34985596 Free PMC article.
-
The modulation of the symbiont/host interaction between Wolbachia pipientis and Aedes fluviatilis embryos by glycogen metabolism.PLoS One. 2014 Jun 13;9(6):e98966. doi: 10.1371/journal.pone.0098966. eCollection 2014. PLoS One. 2014. PMID: 24926801 Free PMC article.
-
Aedes aegypti: egg morphology and embryonic development.Parasit Vectors. 2021 Oct 13;14(1):531. doi: 10.1186/s13071-021-05024-6. Parasit Vectors. 2021. PMID: 34645492 Free PMC article.
-
Eggs of the mosquito Aedes aegypti survive desiccation by rewiring their polyamine and lipid metabolism.PLoS Biol. 2023 Oct 24;21(10):e3002342. doi: 10.1371/journal.pbio.3002342. eCollection 2023 Oct. PLoS Biol. 2023. PMID: 37874799 Free PMC article.
References
-
- Christophers SR. Aedes aegypti (L) The Yellow Fever Mosquito. Its Life History, Bionomics and Structure. Cambridge; Cambridge University Press; 1960.
-
- Kliewer JW. Weight and hatchability of Aedes aegypti eggs. A Entomol Soc Am. 1961;54:912–917.
-
- Clements AN. The biology of mosquitoes. Development, nutrition and reproduction. London; Chapman and Hall; 1992.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

