Functional conservation between rodents and chicken of regulatory sequences driving skeletal muscle gene expression in transgenic chickens
- PMID: 20184756
- PMCID: PMC2841079
- DOI: 10.1186/1471-213X-10-26
Functional conservation between rodents and chicken of regulatory sequences driving skeletal muscle gene expression in transgenic chickens
Abstract
Background: Regulatory elements that control expression of specific genes during development have been shown in many cases to contain functionally-conserved modules that can be transferred between species and direct gene expression in a comparable developmental pattern. An example of such a module has been identified at the rat myosin light chain (MLC) 1/3 locus, which has been well characterised in transgenic mouse studies. This locus contains two promoters encoding two alternatively spliced isoforms of alkali myosin light chain. These promoters are differentially regulated during development through the activity of two enhancer elements. The MLC3 promoter alone has been shown to confer expression of a reporter gene in skeletal and cardiac muscle in transgenic mice and the addition of the downstream MLC enhancer increased expression levels in skeletal muscle. We asked whether this regulatory module, sufficient for striated muscle gene expression in the mouse, would drive expression in similar domains in the chicken.
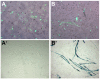
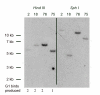
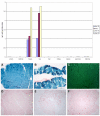
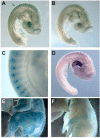
Results: We have observed that a conserved downstream MLC enhancer is present in the chicken MLC locus. We found that the rat MLC1/3 regulatory elements were transcriptionally active in chick skeletal muscle primary cultures. We observed that a single copy lentiviral insert containing this regulatory cassette was able to drive expression of a lacZ reporter gene in the fast-fibres of skeletal muscle in chicken in three independent transgenic chicken lines in a pattern similar to the endogenous MLC locus. Reporter gene expression in cardiac muscle tissues was not observed for any of these lines.
Conclusions: From these results we conclude that skeletal expression from this regulatory module is conserved in a genomic context between rodents and chickens. This transgenic module will be useful in future investigations of muscle development in avian species.
Figures


Similar articles
-
Distinct gene expression patterns in skeletal and cardiac muscle are dependent on common regulatory sequences in the MLC1/3 locus.Mol Cell Biol. 1996 Aug;16(8):4524-34. doi: 10.1128/MCB.16.8.4524. Mol Cell Biol. 1996. PMID: 8754853 Free PMC article.
-
Modular elements of the MLC 1f/3f locus confer fiber-specific transcription regulation in transgenic mice.Dev Genet. 1996;19(2):157-62. doi: 10.1002/(SICI)1520-6408(1996)19:2<157::AID-DVG7>3.0.CO;2-8. Dev Genet. 1996. PMID: 8900048
-
Myosin light chain 3F regulatory sequences confer regionalized cardiac and skeletal muscle expression in transgenic mice.J Cell Biol. 1995 Apr;129(2):383-96. doi: 10.1083/jcb.129.2.383. J Cell Biol. 1995. PMID: 7721942 Free PMC article.
-
Modular regulation of the MLC1F/3F gene and striated muscle diversity.Microsc Res Tech. 2000 Sep 15;50(6):510-21. doi: 10.1002/1097-0029(20000915)50:6<510::AID-JEMT8>3.0.CO;2-1. Microsc Res Tech. 2000. PMID: 10998640 Review.
-
Functions of myosin light chain-2 (MYL2) in cardiac muscle and disease.Gene. 2015 Sep 10;569(1):14-20. doi: 10.1016/j.gene.2015.06.027. Epub 2015 Jun 12. Gene. 2015. PMID: 26074085 Free PMC article. Review.
Cited by
-
En masse lentiviral gene delivery to mouse fertilized eggs via laser perforation of zona pellucida.Transgenic Res. 2018 Feb;27(1):39-49. doi: 10.1007/s11248-017-0056-8. Epub 2018 Feb 13. Transgenic Res. 2018. PMID: 29442214 Free PMC article.
-
Chicken embryo as a model in second heart field development.Heliyon. 2023 Mar 3;9(3):e14230. doi: 10.1016/j.heliyon.2023.e14230. eCollection 2023 Mar. Heliyon. 2023. PMID: 36923876 Free PMC article. Review.
-
Transgenesis and web resources in quail.Elife. 2020 May 27;9:e56312. doi: 10.7554/eLife.56312. Elife. 2020. PMID: 32459172 Free PMC article.
-
Generation of transgenic chickens by the non-viral, cell-based method: effectiveness of some elements of this strategy.J Appl Genet. 2018 Feb;59(1):81-89. doi: 10.1007/s13353-018-0429-6. Epub 2018 Jan 25. J Appl Genet. 2018. PMID: 29372515 Free PMC article. Review.
-
Laser-assisted Lentiviral Gene Delivery to Mouse Fertilized Eggs.J Vis Exp. 2018 Nov 1;(141):10.3791/58327. doi: 10.3791/58327. J Vis Exp. 2018. PMID: 30451224 Free PMC article.
References
-
- Istrail S, De-Leon SB, Davidson EH. The regulatory genome and the computer. Dev Biol. 2007;310:187–195. - PubMed
-
- Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134:25–36. - PubMed
-
- Morrison A, Chaudhuri C, Ariza-McNaughton L, Muchamore I, Kuroiwa A, Krumlauf R. Comparative analysis of chicken Hoxb-4 regulation in transgenic mice. Mech Dev. 1995;53:47–59. - PubMed
-
- Beckers J, Gérard M, Duboule D. Transgenic analysis of a potential Hoxd-11 limb regulatory element present in tetrapods and fish. Dev Biol. 1996;180:543–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

