Neural crest cell-specific deletion of Rac1 results in defective cell-matrix interactions and severe craniofacial and cardiovascular malformations
- PMID: 20184871
- PMCID: PMC2854286
- DOI: 10.1016/j.ydbio.2010.02.021
Neural crest cell-specific deletion of Rac1 results in defective cell-matrix interactions and severe craniofacial and cardiovascular malformations
Abstract
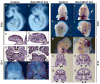
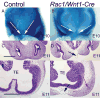
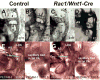
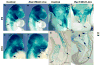
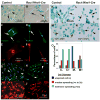
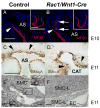
The small GTP-binding protein Rac1, a member of the Rho family of small GTPases, has been implicated in regulation of many cellular processes including adhesion, migration and cytokinesis. These functions have largely been attributed to its ability to reorganize cytoskeleton. While the function of Rac1 is relatively well known in vitro, its role in vivo has been poorly understood. It has previously been shown that in neural crest cells (NCCs) Rac1 is required in a stage-specific manner to acquire responsiveness to mitogenic EGF signals. Here we demonstrate that mouse embryos lacking Rac1 in neural crest cells (Rac1/Wnt1-Cre) showed abnormal craniofacial development including regional ectodermal detachment associated with mesenchymal acellularity culminating in cleft face at E12. Rac1/Wnt1-Cre mutants also displayed inappropriate remodelling of pharyngeal arch arteries and defective outflow tract septation resulting in the formation of a common arterial trunk ('persistent truncus arteriosus' or PTA). The mesenchyme around the aortic sac also developed acellular regions, and the distal aortic sac became grossly dysmorphic, forming a pair of bilateral, highly dilated arterial structures connecting to the dorsal aortas. Smooth muscle cells lacking Rac1 failed to differentiate appropriately, and subpopulations of post-migratory NCCs demonstrated aberrant cell death and attenuated proliferation. These novel data demonstrate that while Rac1 is not required for normal NCC migration in vivo, it plays a critical cell-autonomous role in post-migratory NCCs during craniofacial and cardiac development by regulating the integrity of the craniofacial and pharyngeal mesenchyme.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Inactivation of Cdc42 in neural crest cells causes craniofacial and cardiovascular morphogenesis defects.Dev Biol. 2013 Nov 15;383(2):239-52. doi: 10.1016/j.ydbio.2013.09.013. Epub 2013 Sep 18. Dev Biol. 2013. PMID: 24056078
-
Pinch1 is required for normal development of cranial and cardiac neural crest-derived structures.Circ Res. 2007 Mar 2;100(4):527-35. doi: 10.1161/01.RES.0000259041.37059.8c. Epub 2007 Feb 1. Circ Res. 2007. PMID: 17272814 Free PMC article.
-
Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells.Development. 2004 Jul;131(14):3481-90. doi: 10.1242/dev.01214. Development. 2004. PMID: 15226263
-
Neural crest cells in cardiovascular development.Curr Top Dev Biol. 2015;111:183-200. doi: 10.1016/bs.ctdb.2014.11.006. Epub 2015 Jan 20. Curr Top Dev Biol. 2015. PMID: 25662261 Review.
-
Neural crest contribution to the cardiovascular system.Adv Exp Med Biol. 2006;589:134-54. doi: 10.1007/978-0-387-46954-6_8. Adv Exp Med Biol. 2006. PMID: 17076279 Review.
Cited by
-
TGF-β-activated kinase 1 (Tak1) mediates agonist-induced Smad activation and linker region phosphorylation in embryonic craniofacial neural crest-derived cells.J Biol Chem. 2013 May 10;288(19):13467-80. doi: 10.1074/jbc.M112.431775. Epub 2013 Apr 1. J Biol Chem. 2013. PMID: 23546880 Free PMC article.
-
Glycogen synthase kinase 3 controls migration of the neural crest lineage in mouse and Xenopus.Nat Commun. 2018 Mar 19;9(1):1126. doi: 10.1038/s41467-018-03512-5. Nat Commun. 2018. PMID: 29555900 Free PMC article.
-
Symmetry and fluctuation of cell movements in neural crest-derived facial mesenchyme.Development. 2021 May 1;148(9):dev193755. doi: 10.1242/dev.193755. Epub 2021 May 7. Development. 2021. PMID: 33757991 Free PMC article.
-
Regulation of cell protrusions by small GTPases during fusion of the neural folds.Elife. 2016 Apr 26;5:e13273. doi: 10.7554/eLife.13273. Elife. 2016. PMID: 27114066 Free PMC article.
-
Deletion of integrin-linked kinase from neural crest cells in mice results in aortic aneurysms and embryonic lethality.Dis Model Mech. 2013 Sep;6(5):1205-12. doi: 10.1242/dmm.011866. Epub 2013 Jun 5. Dis Model Mech. 2013. PMID: 23744273 Free PMC article.
References
-
- Aznar S, Lacal JC. Rho signals to cell growth and apoptosis. Cancer Lett. 2001;165:1–10. - PubMed
-
- Barlow LA. Cranial nerve development: placodal neurons ride the crest. Curr Biol. 2002;12:R171–173. - PubMed
-
- Breau MA, Dahmani A, Broders-Bondon F, Thiery JP, Dufour S. {beta}1 integrins are required for the invasion of the caecum and proximal hindgut by enteric neural crest cells. Development. 2009;136:2791–2801. - PubMed
-
- Brown CB, Baldwin HS. Neural crest contribution to the cardiovascular system. Adv Exp Med Biol. 2006;589:134–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

