Alphavirus replicon-based enhancement of mucosal and systemic immunity is linked to the innate response generated by primary immunization
- PMID: 20184975
- PMCID: PMC2855431
- DOI: 10.1016/j.vaccine.2010.02.010
Alphavirus replicon-based enhancement of mucosal and systemic immunity is linked to the innate response generated by primary immunization
Abstract
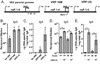
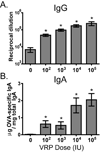
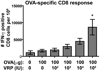
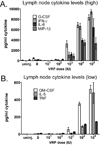
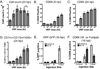
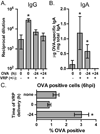
Venezuelan equine encephalitis virus replicon particles (VRP) function as an effective systemic, cellular and mucosal adjuvant when codelivered with antigen, and show promise for use as a component in new and existing human vaccine formulations. We show here that VRP are effective at low dose and by intramuscular delivery, two useful features for implementation of VRP as a vaccine adjuvant. In mice receiving a prime and boost with antigen, we found that VRP are required in prime only to produce a full adjuvant effect. This outcome indicates that the events triggered during prime with VRP are sufficient to establish the nature and magnitude of the immune response to a second exposure to antigen. Events induced by VRP in the draining lymph node after prime include robust secretion of many inflammatory cytokines, upregulation of CD69 on leukocytes, and increased cellularity, with a disproportionate increase of a cell population expressing CD11c, CD11b, and F4/80. We show that antigen delivered 24h after administration of VRP does not benefit from an adjuvant effect, indicating that the events which are critical to VRP-mediated adjuvant activity occur within the first 24h. Further studies of the events induced by VRP will help elucidate the mechanism of VRP adjuvant activity and will advance the safe implementation of this adjuvant in human vaccines.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

References
-
- Kwissa M, Kasturi SP, Pulendran B. The science of adjuvants. Expert review of vaccines. 2007 Oct;6(5):673–684. - PubMed
-
- McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007 Nov;27(5):687–690. - PubMed
-
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. - PubMed
-
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nature reviews. 2006 Feb;6(2):148–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

