A complex 3D human tissue culture system based on mammary stromal cells and silk scaffolds for modeling breast morphogenesis and function
- PMID: 20185172
- PMCID: PMC2847607
- DOI: 10.1016/j.biomaterials.2010.01.118
A complex 3D human tissue culture system based on mammary stromal cells and silk scaffolds for modeling breast morphogenesis and function
Abstract
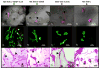
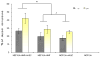
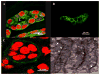
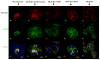
Epithelial-stromal interactions play a crucial role in normal embryonic development and carcinogenesis of the human breast while the underlying mechanisms of these events remain poorly understood. To address this issue, we constructed a physiologically relevant, three-dimensional (3D) culture surrogate of complex human breast tissue that included a tri-culture system made up of human mammary epithelial cells (MCF10A), human fibroblasts and adipocytes, i.e., the two dominant breast stromal cell types, in a Matrigel/collagen mixture on porous silk protein scaffolds. The presence of stromal cells inhibited MCF10A cell proliferation and induced both alveolar and ductal morphogenesis and enhanced casein expression. In contrast to the immature polarity exhibited by co-cultures with either fibroblasts or adipocytes, the alveolar structures formed by the tri-cultures exhibited proper polarity similar to that observed in breast tissue in vivo. Only alveolar structures with reverted polarity were observed in MCF10A monocultures. Consistent with their phenotypic appearance, more functional differentiation of epithelial cells was also observed in the tri-cultures, where casein alpha- and -beta mRNA expression was significantly increased. This in vitro tri-culture breast tissue system sustained on silk scaffold effectively represents a more physiologically relevant 3D microenvironment for mammary epithelial cells and stromal cells than either co-cultures or monocultures. This experimental model provides an important first step for bioengineering an informative human breast tissue system, with which to study normal breast morphogenesis and neoplastic transformation.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Preadipocytes stimulate ductal morphogenesis and functional differentiation of human mammary epithelial cells on 3D silk scaffolds.Tissue Eng Part A. 2009 Oct;15(10):3087-98. doi: 10.1089/ten.TEA.2008.0670. Tissue Eng Part A. 2009. PMID: 19338449 Free PMC article.
-
Hormone-responsive 3D multicellular culture model of human breast tissue.Biomaterials. 2012 Apr;33(12):3411-20. doi: 10.1016/j.biomaterials.2012.01.011. Epub 2012 Feb 4. Biomaterials. 2012. PMID: 22309836 Free PMC article.
-
Milk protein expression and ductal morphogenesis in the mammary gland in vitro: hormone-dependent and -independent phases of adipocyte-mammary epithelial cell interaction.Dev Biol. 1987 Mar;120(1):245-58. doi: 10.1016/0012-1606(87)90122-9. Dev Biol. 1987. PMID: 3817293
-
Apical polarity in three-dimensional culture systems: where to now?J Biol. 2010;9(1):2. doi: 10.1186/jbiol213. Epub 2010 Jan 21. J Biol. 2010. PMID: 20092610 Free PMC article. Review.
-
Myoepithelial cells: their origin and function in breast morphogenesis and neoplasia.J Mammary Gland Biol Neoplasia. 2005 Jul;10(3):261-72. doi: 10.1007/s10911-005-9586-4. J Mammary Gland Biol Neoplasia. 2005. PMID: 16807805 Free PMC article. Review.
Cited by
-
Fabrication and Characterization of a Three-Dimensional Fibrin Gel Model to Evaluate Anti-Proliferative Effects of Astragalus hamosus Plant Extract on Breast Cancer Cells.Asian Pac J Cancer Prev. 2022 Feb 1;23(2):731-741. doi: 10.31557/APJCP.2022.23.2.731. Asian Pac J Cancer Prev. 2022. PMID: 35225487 Free PMC article.
-
In Vitro 3D Cultures to Model the Tumor Microenvironment.Cancers (Basel). 2021 Jun 13;13(12):2970. doi: 10.3390/cancers13122970. Cancers (Basel). 2021. PMID: 34199324 Free PMC article. Review.
-
Stem cell maintenance in a different niche.Clin Exp Reprod Med. 2013 Jun;40(2):47-54. doi: 10.5653/cerm.2013.40.2.47. Epub 2013 Jun 30. Clin Exp Reprod Med. 2013. PMID: 23875159 Free PMC article.
-
Gene expression analysis of in vitro cocultures to study interactions between breast epithelium and stroma.J Biomed Biotechnol. 2011;2011:520987. doi: 10.1155/2011/520987. Epub 2011 Dec 13. J Biomed Biotechnol. 2011. PMID: 22203785 Free PMC article. Review.
-
Synthetic adipose tissue models for studying mammary gland development and breast tissue engineering.J Mammary Gland Biol Neoplasia. 2010 Sep;15(3):365-76. doi: 10.1007/s10911-010-9192-y. Epub 2010 Sep 12. J Mammary Gland Biol Neoplasia. 2010. PMID: 20835885 Review.
References
-
- Mettlin C. Global breast cancer mortality statistics. CA Cancer J Clin. 1999;49:138–144. - PubMed
-
- Thomsen A, Kolesar JM. Chemoprevention of breast cancer. Am J Health Syst Pharm. 2008;65:2221–2228. - PubMed
-
- Howlett AR, Bissell MJ. The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithelial Cell Biol. 1993;2:79–89. - PubMed
-
- Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

