Ricinus communis agglutinin I leads to rapid down-regulation of VEGFR-2 and endothelial cell apoptosis in tumor blood vessels
- PMID: 20185574
- PMCID: PMC2843481
- DOI: 10.2353/ajpath.2010.090561
Ricinus communis agglutinin I leads to rapid down-regulation of VEGFR-2 and endothelial cell apoptosis in tumor blood vessels
Abstract
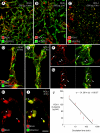
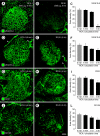
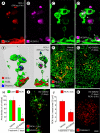
Ricinus communis agglutinin I (RCA I), a galactose-binding lectin from castor beans, binds to endothelial cells at sites of plasma leakage, but little is known about the amount and functional consequences of binding to tumor endothelial cells. We addressed this issue by examining the effects of RCA I on blood vessels of spontaneous pancreatic islet-cell tumors in RIP-Tag2 transgenic mice. After intravenous injection, RCA I bound strongly to tumor vessels but not to normal blood vessels. At 6 minutes, RCA I fluorescence of tumor vessels was largely diffuse, but over the next hour, brightly fluorescent dots appeared as the lectin was internalized by endothelial cells. RCA I injection led to a dose- and time-dependent decrease in vascular endothelial growth factor receptor-2 (VEGFR-2) immunoreactivity in tumor endothelial cells, with 95% loss over 6 hours. By comparison, VEGFR-3, CD31, and CD105 had decreases in the range of 21% to 33%. Loss of VEGFR-2 was followed by increased activated caspase-3 in tumor vessels. Prior inhibition of VEGF signaling by AG-028262 decreased RCA I binding and internalization into tumor vessels. These findings indicate RCA I preferentially binds to and is internalized by tumor endothelial cells, which leads to VEGFR-2 down-regulation, endothelial cell apoptosis, and tumor vessel regression. Together, the results illustrate the selective impact of RCA I on VEGF signaling in tumor blood vessels.
Figures




Similar articles
-
Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts.Am J Pathol. 2004 Jul;165(1):35-52. doi: 10.1016/S0002-9440(10)63273-7. Am J Pathol. 2004. PMID: 15215160 Free PMC article.
-
VEGFR-1 mediates endothelial differentiation and formation of blood vessels in a murine model of infantile hemangioma.Am J Pathol. 2011 Nov;179(5):2266-77. doi: 10.1016/j.ajpath.2011.07.040. Epub 2011 Sep 21. Am J Pathol. 2011. PMID: 21945324 Free PMC article.
-
VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature.Am J Physiol Heart Circ Physiol. 2006 Feb;290(2):H560-76. doi: 10.1152/ajpheart.00133.2005. Epub 2005 Sep 19. Am J Physiol Heart Circ Physiol. 2006. PMID: 16172168
-
Regional effects of an antivascular endothelial growth factor receptor monoclonal antibody on receptor phosphorylation and apoptosis in human 253J B-V bladder cancer xenografts.Cancer Res. 2004 Jul 1;64(13):4601-10. doi: 10.1158/0008-5472.CAN-2879-2. Cancer Res. 2004. PMID: 15231672
-
The vascular endothelial growth factor (VEGF)/VEGF receptor 2 pathway is critical for blood vessel survival in corpora lutea of pregnancy in the rodent.Endocrinology. 2005 Mar;146(3):1301-11. doi: 10.1210/en.2004-0765. Epub 2004 Dec 9. Endocrinology. 2005. PMID: 15591152
Cited by
-
Anti-tumor innate immunity activated by intermittent metronomic cyclophosphamide treatment of 9L brain tumor xenografts is preserved by anti-angiogenic drugs that spare VEGF receptor 2.Mol Cancer. 2014 Jun 26;13:158. doi: 10.1186/1476-4598-13-158. Mol Cancer. 2014. PMID: 24965046 Free PMC article.
-
In situ force mapping of mammary gland transformation.Integr Biol (Camb). 2011 Sep;3(9):910-21. doi: 10.1039/c1ib00043h. Epub 2011 Aug 15. Integr Biol (Camb). 2011. PMID: 21842067 Free PMC article.
-
Collagen VI ablation retards brain tumor progression due to deficits in assembly of the vascular basal lamina.Am J Pathol. 2012 Mar;180(3):1145-1158. doi: 10.1016/j.ajpath.2011.11.006. Epub 2011 Dec 23. Am J Pathol. 2012. PMID: 22200614 Free PMC article.
-
3'-Sulfo-TF Antigen Determined by GAL3ST2/ST3GAL1 Is Essential for Antitumor Activity of Fungal Galectin AAL/AAGL.ACS Omega. 2021 Jul 1;6(27):17379-17390. doi: 10.1021/acsomega.1c01544. eCollection 2021 Jul 13. ACS Omega. 2021. PMID: 34278124 Free PMC article.
-
Reduced VEGF production, angiogenesis, and vascular regrowth contribute to the antitumor properties of dual mTORC1/mTORC2 inhibitors.Cancer Res. 2011 Mar 1;71(5):1573-83. doi: 10.1158/0008-5472.CAN-10-3126. Cancer Res. 2011. PMID: 21363918 Free PMC article.
References
-
- Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102–111. - PubMed
-
- McDonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res. 2002;62:5381–5385. - PubMed
-
- Nishida S, Akai F, Hiruma S, Maeda M, Tanji K, Hashimoto S. Experimental study of WGA binding on the endothelial cell surface in cerebral ischemia. Histol Histopathol. 1986;1:69–74. - PubMed
-
- Jackson CJ, Garbett PK, Nissen B, Schrieber L. Binding of human endothelium to Ulex europaeus I-coated Dynabeads: application to the isolation of microvascular endothelium. J Cell Sci. 1990;96:257–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

